Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.4 Madrid jul./ago. 2011
Impact of different protein sources in the glycemic and insulinemic responses
Impacto de diferentes fuentes proteicas en la respuesta glucémica e insulinémica
F. C. Esteves de Oliveira1, A. C. Pinheiro Volp2 and R. C. Alfenas3
1Candidate for doctoral degree in Food Science and Technology. Federal University of Viçosa. Minas Gerais. Brazil.
2Assistant Professor. Nutrition School. Federal University of Ouro Preto. Minas Gerais. Brazil.
3Assistant Professor. Nutrition and Health Department. Federal University of Viçosa. Minas Gerais. Brazil.
ABSTRACT
Objective: The maintenance of normal blood glucose concentrations is a crucial factor to the achievement of a good health status throughout life. However, the occurrence of abnormalities in this parameter has become increasingly common, which can result in several non-transmissible diseases, such as type 2 diabetes and cardiovascular diseases. Therefore, the purpose of this study was to discuss the role of protein sources in the glycemic and insulinemic responses.
Methods: In this review paper, we critically analyzed recently published studies that discussed the role of different protein sources in the glycemic and insulinemic responses in healthy individuals and in those who have cardiovascular diseases or type 2 diabetes.
Results: The results of some of these studies suggest that the daily ingestion of at least one high protein meal containing low to moderate amounts of carbohydrate increases insulin secretion and glucose uptake, improving insulin sensitivity. Furthermore, the results indicate that these effects are particularly associated with the consumption of animal protein (p.e. hydrolyzed whey protein), which has a high content of branched-chain amino acids such as leucine, valine and others such as arginine, which leads to improvements in insulin secretion and uptake glucose, since it increases insulin sensitivity. However, there is still no consensus in the literature about the quantity and quality of protein capable of reducing or maintaining blood-glucose concentrations at the desirable range, without causing adverse effects. The difference in the results of the studies may be associated to methodological problems presented by these studies.
Conclusions: Well designed studies should be conducted to identify the quantity and quality of protein that can lead to the improvement on blood glucose concentrations, without negative effects to health. These studies should also identify the mechanisms and the magnitude by which protein may affect glycemic response.
Key words: Protein quality. Glycemic response. Type 2 diabetes. Cardiovascular diseases.
RESUMEN
Objetivo: El mantenimiento de la glucemia en concentraciones normales representa un factor crucial para conseguir un buen estado de salud a lo largo de la vida. Sin embargo, en la actualidad, cada vez son más frecuentes las alteraciones de dicho parámetro, siendo un factor de riesgo potencial para el desarrollo de diabetes del tipo 2 y enfermedades cardiovasculares. En este sentido, el objetivo del presente trabajo fue discutir el papel de diferentes fuentes proteicas en la repuesta glucémica y insulinémica a la alimentación.
Metodología: Se ha realizado una revisión crítica de la literatura reciente teniendo en cuenta aquellos estudios que se han centrado en el análisis del papel de las diferentes fuentes proteicas en la respuesta glucémica y insulinémica tanto en individuos sanos como en aquellos que ya han desarrollado alguna alteración cardiovascular o bien presentan diabetes mellitus tipo 2.
Resultados: Los resultados de los estudios científicos revisados sugieren que la ingesta de una o más comidas con alto contenido en proteínas y un contenido bajo o moderado de hidratos de carbono, mejora la secreción de insulina y la captación de glucosa, mejorando así la sensibilidad insulínica. Además, la fuente proteica (origen animal o vegetal) y la composición aminoacídica de las proteínas ingeridas juegan a su vez un papel importante ya que pueden dar lugar a efectos diferenciados en la glucemia y insulinemia. Así, normalmente aquellos estudios que han utilizado alimentos cuya composición proteica se basa principalmente en aminoácidos de cadena ramificada como la leucina, valina y otros como la arginina, han demostrado mejoras en la secreción de insulina y captación de glucosa, favoreciendo una mayor sensibilidad a la hormona. Además, este efecto se encuentra particularmente asociado al consumo de proteínas de origen animal (como las proteínas hidrolizadas del suero de leche), las cuales presentan un alto contenido de aminoácidos esenciales de cadena ramificada.
Conclusiones: Sin embargo, todavía no hay un consenso respecto a la cantidad y calidad proteica capaz de reducir o bien mantener en equilibrio homeostático la concentración de glucosa con el fin de conseguir un buen estado de salud sin llegar a producir efectos adversos.
Palabras clave: Calidad proteica. Glucemia. Diabetes mellitus del tipo 2. Enfermedades cardiovasculares.
Abbreviations
CVD: Cardiovascular diseases.
GIP: Glucose-dependent insulinotropic polypeptide.
GLP-1: Glucagon-like Polypeptide-1.
kg: Kilogram.
g: Gram.
BMI: Body mass index.
kg/m2: Kilogram/square meter.
%: Percentage.
mv: milivolts.
BCAA: Branched-chain amino acids.
LEU: Leucine.
CHO: Carbohydrate.
PTN: Protein.
DM2: Type 2 Diabetes.
PEPCK: Phosphoenolpiruvate carboxykinase.
PGC-1α: Receptor and co-activator 1 α of activated reproducer of peroxisome.
β: Beta.
g/kg/h: Gram/kilogram/hour.
g/L: Gram/liter. mmol: milimol.
LIP: Lipid.
HDL-c: High Density Lipoprotein-cholesterol.
LDL-c: Low Density Lipoprotein-cholesterol.
Introduction
The maintenance of blood glucose concentration at the normal range is important to health throughout life. However, the occurrence of abnormalities in this parameter has become very frequent, leading to the metabolic syndrome and the development of several chronic diseases, such as type 2 diabetes (DM2) and cardiovascular diseases (CVD).1,23 It has been claimed that the level of physical activity, body composition and some characteristics presented by food (For example: fruit ripeness, food physical form, processing method and preparation time, macronutrient contents, etc.) may significantly affect the postprandial glycemic response.2,4,5
Scientific evidences suggest that the ingestion of high-protein meal, presenting low to moderate quantities of carbohydrate increases insulin secretion.6 This effect may be attributed to the synergistic effect associated to increased protein and reduced carbohydrate ingestion, which result in the improvement of insulin sensitivity and in glucose uptake. Besides being potent insulin secretagogues (valine, leucine, isoleucine), some amino acids stimulate the incretin system: Glucose-dependent insulinotropic polypeptide (GIP) and Glucagon-like Polypeptide-1 (GLP-1), thus reducing the speed of gastric leakage, stimulating insulin secretion and inhibiting glucagon secretion.7,8,9,10,11
Furthermore, some authors suggest that the source (animal or vegetable) and the amino acid composition of proteins may also cause different effects on blood glucose concentration.12 Therefore, the present study aimed at discussing the role of different protein sources on glycemic and insulinemic responses, after critical analysis of studies on this subject already published.
Proteins and Glycemic Response
The intake of normal quantities of protein (0.8 g protein/kg/day, according to recommendations of the Institute of Medicine13) stimulates insulin secretion, and may reduce significantly blood glucose concentration, depending on the amino acid profile presented by the protein ingested. This insulinotropic effect is also frequently observed after the consumption of high quantities of proteins.12,14 However, there is no consensus in the literature about how much protein would cause such effect, without affecting health. A study involving normal weight individuals verified that the insulinemic response curve was higher as the protein intake and glycemic response curve were lower in response to the consumption of breakfast containing protein/glucose (g) (50/0, 0/50, 10/50, 30/50, 50/50). However, the gradual increase in protein intake did not result in a significant increase in insulinemic response.15
In type 2 diabetic people, the increase in insulin secretion stimulation may be beneficial to hyperglycemia reduction, preventing the occurrence of lipolysis and excessive release of fatty acids, thus avoiding the occurrence of problems related to CVD.16 In a previous study involving non-treated type 2 diabetics, which used the same protocol adopted by the authors of the above mentioned study,15 the area under the insu-linemic response curve increased linearly and the glycemic response decreased according to the quantity of protein ingested. This demonstrates that insulin secretion response is much more sensitive to protein intake by diabetic people.17 However, it is important to emphasize that, in a long term, the excessive intake of proteins may lead to the occurrence of renal overload, development of CVD and osteoporosis.18,19,20
Effects on the regulation of blood glucose concentrations
The insulinotropic effect presented by proteins may lead to significant increase (over 200%) in insulinemic response and glucose uptake. This effect has been observed specially for the consumption of animal protein (such as whey hydrolyzed protein), which presents high amount of essential branched-chain amino acids.16,21
Gannon et al.6 verified that the intake of a high-protein (30% of protein) and low-carbohydrate (40% of carbohydrate) diet, during five weeks, reduced the postprandial blood glucose concentration in type 2 diabetic people and improved the glycemic control, if compared to the control diet (15% of protein, 55% of carbohydrate, and 30% of lipid). It is worth stressing that in this stud,6 the consumption of high-protein diet during five weeks did not affect the release of creatinin and urinary microalbumin, which are indicators of renal function. In spite of the importance of such results, it cannot affirm that such parameters would remain unchanged if such diet were applied for a long period. Furthermore, the participants of such study did not present homogeneous characteristics of age (39-79 years-old) or body mass index (BMI of 22-37 kg/m2), which may have influenced the results. It has been observed that individuals at the age of 60 years and/or BMI higher than 24.99 kg/m2 could have reduced insulin secretion and tolerance to the blood glucose level. Anther factor to be considered is that the effect observed in the blood glucose level was due to the increased protein intake or reduced carbohydrate in the diet, or a synergetic effect of both factors.
Weigle et al.14 verified a greater stimulus for insulin secretion after two weeks of eucaloric high-protein diet consumption (30% protein, 20% lipid and 50% carbohydrate) compared to the control diet (15% protein, 35% lipid and 50% carbohydrate) in overweight and obese individuals. This effect was attributed to the higher ability of proteins to stimulate insulin secretion, in comparison to lipids. In this work, the quantities of carbohydrates remained constant in the tested diets in order to isolate the insulinotropic effect of protein. But, since this evaluation was not carried out in this study,14 the glycemic response could not be inferred from these individuals.
It was evaluated the dose-response effect of 0 to 30 g (0, 5, 10 or 30 g) of soy protein concentrate or maize oil on glycemic response and insulin sensitivity, after the intake of 50 g of glucose, in non-diabetic individuals (normal insulinemic and hyperinsulinemic). The consumption of the meals tested did not affect the average blood glucose level in the groups of normal insulinemic and hyperinsulinemic individuals. However, it was observed a higher reduction (p < 0.05) in the glycemic response when the dose of 30 g of protein was ingested.22 This is an interesting result, but it must be emphasized that the meals tested in this study were liquid. Liquid food does not need chewing, and requires less time to pass through the intestine. Besides, its nutrients are more bioaccessible and bioavailable,23 which could lead to a bigger postprandial glycemic elevation. Thus, it cannot be determined if these results could be inferred for solid food.
Some authors24,25 suggest that different proteins may stimulate the release of insulin differently. The insulinotropic effect of amino acids may occur because they allow the entrance of calcium, by a voltage-dependent mechanism related to the depolarization of the cell membrane. This depolarization occurs when there is a reduction in cell potassium exit, followed by the opening of sodium channels, leading to an intracellular increase of sodium and a reduction of calcium efflux. Consequently, the difference in the membrane potential reaches 0 mv. Thus, the channels of dependent voltage calcium open up themselves, promoting an increase in the cytoplasmatic calcium concentration, which carries on to a maximum insulin secretion. Other possible mechanisms of amino acid actions also occur to the blood glucose level, in other words, the stimulus to insulin secretion does not occur by fixation of glucose to a membrane receptor, but by a receptor that would be an enzyme of its own metabolism. For example, the insulinotropic effects of leucine seems be related to the glutamate dehydrogenase and dehydrogenase of branched keto acids.24,25
Some authors26 consider that leucine stimulates insulin secretion, acting both as a metabolic substrate and allosteric activator of the enzyme glutamate dehydrogenase, leading to increased glutaminolysis. Glutamate dehydrogenase is the key enzyme controlling aminoacids and ammonia metabolism in pancreatic β cells, liver, and brain. It is believed that leucine or its transaminated product, a-ketoisocaproate, regulates KATP channel activity and results in increased free cytosolic Ca2+, which triggers insulin secretory granule exocytosis via mechanisms involving the activation of some protein kinases and protein acylation.
Another possible mechanism by which leucine can stimulate insulin secretion is the regulation of gene transcription and protein synthesis in pancreatic islet β cells through the activation of the protein serine-threonine (mammalian target of rapamycin-mTOR). The activation of this protein significantly stimulates the phosphorylation of p70S6K and increases protein synthesis in pancreatic β cells in a rapamycin-sensitive and insulin-independent manner at physiological concentrations ranging from 0.4 mM to 4 mM.26
It is known that high concentrations of branched-chain amino acids (BCAA) contribute to the production of glucose in the liver (gluconeogenesis), through the alanine-glucose cycle. The degradation of these amino acids in the skeletal muscles is connected to the production of alanine and glutamine, and the maintenance of glycemic homeostasis. This cycle involves a continuous release of BCAA from the liver and splenic circulation to the skeletal muscle. The amino acid gain by the muscular tissue leads to intracellular concentration increase and stimulates BCAA transamination for alanine production. This amino acid is released from the muscle to the blood and is captured by the liver to participate in the gluconeogenesis, which contributes to the homeostasis of the blood glucose level. The amino acids arising from the alanine-glucose cycle, which serve as the main carbon sources for endogenous production of glucose, represent about 40% of this production during the extended exercise and approximately 70% after nocturnal fasting.27
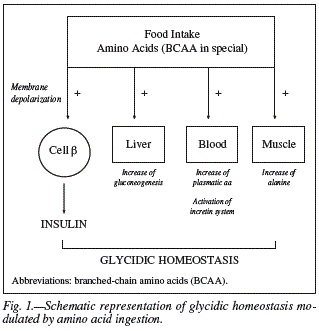
Furthermore, it has been verified the occurrence of a positive correlation between the postprandial insulinemia and the increase of amino acid levels in the plasma. This effect is more intense in response to the presence of some specific amino acids, such as: leucine, valine, lysine and isoleucine. This fact can be explained by their structure, making digestion easier and resulting in faster release of peptides or amino acids bioactive in the bloodstream, where incretins are activated.9,10,21 Therefore, the postprandial metabolic pathway of amino acids could be a crucial step for the insulinotropic properties of dietary proteins. A schematic view of such effects could be observed in the figure 1.
The effects of the hydrolyzed casein and leucine (LEU) on insulinemia and blood glucose concentration were evaluated after the intake of three beverages with different contents: 0.7 g/kg of carbohydrate (CHO) or 0.7 g/kg of CHO + 0.3 g/kg of protein (PTN) or 0.7 g/kg of CHO + 0.3 g/kg PTN + 0.1 g/kg LEU. The beverages were offered to type 2 diabetic people and healthy controls in three different events, with an interval of at least seven days between the tests. It was verified a significant increase in the plasma insulin response after the consumption of beverages CHO + PTN and CHO + PTN + LEU, corresponding to 141 and 204% in the diabetic individuals. In the control individuals, this increase was 66 and 221% respectively, if compared with the beverage that contains CHO (p < 0.05) only. There was a significant reduction of 12 and 15% for the glycemic response in the group with DM2 and 92, and 97%, in the controls after the consumption of beverages CHO + PTN and CHO + PTN + LEU, respectively (p < 0.05). Although the insulinemic response is not different among the experimental groups, the blood glucose concentration was substantially higher in diabetic patients in all classifications, if compared to the controls (p < 0.01).25 The results of this study indicate that the ingestion of proteins with or without leucine increases the insulin secretion in diabetic and non-diabetic individuals, and lead to a reduced glycemic response of these individuals.
Another study evaluated the effects of increased leucine intake in the diet, during 14 weeks, on the improvement of the glycemic control of rats. It was verified that leucine supplementation significantly prevented the hyperglycemia observed after the consumption of a high-fat diet, which is related to the improvement in insulin sensitivity and an increase in glucose tolerance, reduced glucagon concentrations and gene expressions of glucose-6-phosphatase, phosphoenolpiruvate car-boxykinase (PEPCK) and peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α) involved in hepatic gluconeogenesis.28
Nilsson et al.9 evaluated the effect of different protein sources (cod fish, milk, whey, cheese) on insulin secretion in healthy individuals. The quantities of lactose were equivalent to those in dairy meals. Dairy products, mainly whey, presented more insulinotropic properties than other proteins probably because the amino acid composition of such food presents a great quantity of branched-chain amino acids, which are potent insulin secretagogue.9,10,21
In a study29 involving type 2 diabetic people, it was evaluated the effect of adding whey, in high glycemic index meals on insulin secretion and glycemic control. The evaluation occurred on two different days, with at least one-week interval, for each person. Two reference meals were ingested (breakfast - bread, ham and lactose; lunch - mashed potatoes, meat cakes, ham and lactose) on the same day, with an interval of four hours between them. After one-week interval, the individuals received two test meals, which were similar to the reference meals, but replaced the protein source by whey. The reference and test meals were isocaloric. In the end of the experiment, it was verified increased insulinemic response both after breakfast (31%) and after lunch (57%) with the addition of whey. According to the authors, the most insulinotropic effect observed in breakfast could be related to the fact that resistance to insulin is higher in the morning, after a nocturnal fasting, resulting in lower reduction in blood glucose after the first meal. In addition, the quantity of carbohydrate provided at lunch was lesser than in breakfast. It was verified more GIP secretion after the intake of whey. However, the treatments applied in this study did not affect the GLP-1 concentrations. Therefore, these results demonstrate that whey is potentially able to reduce glycemic response throughout the day and, according to the authors, it could be indicated for the treatment of DM2.29
However, it is worth to point out that the participants of that study29 presented a wide range of BMI (26.2 ± 3.1 kg/m2) and age (27-69 years). Furthermore, it was not described for how long they had been diabetic. All of these factors could cause changes in insulin secretion and tolerance to glucose. Increased body fat may lead to hyperinsulinemia or hyperglycemia and aging may be related to reduced secretion of this hormone.30,31,32 It is also very important to consider the time of the disease to evaluate the degree of compromise of pancreatic β cells and the organism's capacity to maintain the homeostasis of blood glucose concentration.
Akhavan et al.33 described the effect of beverages containing different amounts of whey protein or of a single amount of hydrolyzed whey protein associated with different quantities of whey protein in blood glucose and insulin concentrations in healthy young adults. These beverages were consumed 30 minutes before the ad libitum consumption of pizza (experiment 1) or the ingestion of a meal containing a fixed amount of pizza (12 kcal/kg, experiment 2). The results of that study indicated that the consumption of relatively small quantities of whey protein before a meal reduces postprandial glucose and insulin responses. Although the mechanism responsible for this effect is still unclear, it may be explained by the effect of protein on gastric emptying, reducing the postprandial concentrations of glucose and insulin.
In another study,34 the insulinotropic effect of two milk protein fractions, casein and whey protein, intrinsically labeled with L-[1-13C] Leucine was evaluated. These proteins present high content of BCAA, differing only in physical-chemical properties. It was verified that both protein fractions increased plasma insulin concentrations. In this study, the authors report that it is unlikely that this insulinotropic effect is associated with biodisponibility and the presence of a balanced score of amino acids, because casein and whey protein were given in large amounts and have a high digestibility and a well balanced amino acid score.34 However, it is know that casein coagulates when it reaches the stomach, and its digestion is slower, resulting in slower release of amino acids into the circulation and lower oxidation of plasma amino acids, with smaller increase in protein synthesis and increase in inhibition of protein breakdown than what is observed after whey consumption. On the other hand, whey does not coagulate and is digested quickly. Thus, there is a faster release of its amino acids in the blood, resulting in a stronger plasma insulin response.9,29
Van Loon et al.12 performed a crossover double blind study, involving 8 normal weight men, with average age of 21 ± 0.4 years old, to evaluate the insulinotropic effect of the amino acid or protein mixture co-ingested with carbohydrate. In each experimental section, after 12 hours of fasting, ten different beverages were ingested in random sequence, containing 0.8 g/kg/h of carbohydrate and 0.4 g/kg/h of an amino acid or hydrolyzed protein mixture. It was verified that the insulinemic response corresponded positively with the leucine, fenilalanin and tirosin concentration. Still, it was evidenced that the protein mixture of hydrolyzed wheat, free leucine, fenilalanin and carbohydrate increased significantly the insulin concentration. However, it is worth stressing that amino acids and proteins in the isolated form should be used with caution. In this work, adverse effects were observed in the participants, such as several diarrheas, mainly after the administration of 57.1 g/L arginine. The authors suggest that such gastrointestinal symptoms seemed to reduce the intestinal uptake of arginine, because fewer plasma concentrations of arginine were observed after uptake of beverages with higher content of this amino acid.
According to some authors, GIP is secreted at the intestine in response to carbohydrate, lipid and protein intakes.7,29,35 However, Nordt et al.36 did not observe any effect on the GIP's response in type 2 diabetics after intake of high-protein diet.
Johnston & Buller8 evaluated the effect of peanut products as complementary food for the reduction of postprandial protein. Eleven healthy individuals participated of this study (ten women and one man), with BMI 22.7 ± 1.0 and 27.9 ± 2.9 years of age. These participants ate two test meals (bagel and juice or chicken and rice) with and without addition of peanut, in a randomized crossover experimental design. It was observed that the consumption of peanut reduced the postprandial blood glucose. This effect was attributed to the high content of arginine of this oleaginous, which is a potent secretagogue of insulin. However, it must also be considered that peanuts present high contents of lipids (about 50%, mainly monounsaturated) and fibers. These compounds may influence the glycemic response by slowing gastric emptying and/or activating the incretine system with consequent reduction of glucose uptake.6,38,39 Furthermore, it is believed that the physical form of peanuts impedes the complete breaking/grinding of grains by chewing, which affects the digestive process. According to some authors, the absence of rupture of the fibrous cell walls by mechanical processes (chewing), enzymatic and bacteriological affects the bioaccessibility.39,40
According to a review by Brito and Volp,41 arginine was considered the most potent insulin secretagogue among other nine supplemented amino acids. However, Gannon et al.42 in a research involving nine healthy individuals (5 men and 4 women, 21 to 52 years of age), which ingested at 08:00 AM 1 mmol arginine/kg of lean mass or 1 mmol arginine/kg of lean mass + 25 g glucose or 25 g glucose alone or just water, in random order, on separate occasions. It was observed that arginine did not stimulate the secretion of insulin, but it was verified that this amino acid reduced the increase of blood glucose when administrated together with glucose. A difference in gastric emptying rate could be a possible explanation for these results. However, the mechanism responsible for such effect remains unclear, since gastric emptying time was similar after ingestion of glucose alone or arginine plus glucose. It should be emphasized that the concentration of arginine (1 mmol arginine/kg of lean mass) ingested approached the content of a high-protein meal, which may not reflect a habitual intake.
In a recently published study,43 the authors proposed a new index (Food insulinemic index-FII) for the treatment of diabetes. This new tool considers the effect of fat and protein on insulin secretion, since these nutrients can affect blood glucose and are not considered in the daily treatment of diabetes. The study verified that the consumption of mixed meals presenting similar contents of carbohydrate produced different insulinemic responses in healthy subjects. It was observed that low-fat and high-protein meals lead to higher insulinemic response. This result corroborates those observed in the other studies and demonstrates that amino acids are potent insulin secretion stimulators,12,42 especially after the consumption of meals with high carbohydrate and low fat content. However, according to the authors, more research is still needed to validate the concept of FII and to evaluate its ability to predict the relationship between diet and disease.
Possible adverse effects
The increase in protein consumption has been considered a new alternative for the treatment of diabetes mellitus. However, it must be considered that there may be consequences related to the long term protein supply. The increased consumption of food of animal origin, the main source of this macronutrient, is usually connected to higher intake of lipids, mainly saturated, and cholesterol and a lower intake of fibers, which could result in increased CVD risk.18,44
Furthermore, protein intake above the organic needs leads to increased catabolic reactions of its amino acids, increasing the production of byproducts (urea, adenosine triphosphate, carbonic gas, glucose, acetyl Coenzyme A and ketone bodies). Some of them may bring adverse effects to the organism, such as renal overcharge, blood ketosis and CVD.45,46 Therefore, the recommendation of high-protein diet intake by diabetic individuals has still been discussed by researches.
Verhoef et al.18 conducted a randomized, controlled, crossover trial that involved twenty health men, aged 18-44 years, which were kept under free-living conditions, and were divided into two groups. In that study, each man underwent two dietary treatments: a highprotein diet and a low-protein diet. Each treatment period lasted 8 days, and the intervening washout period, during which the men consumed their habitual diet, lasted 13 days. After the washout period, the treatments were reversed. It was verified that the high-protein diet (21% of protein) led to increased homocysteine plasma concentrations throughout the day and after 1 week of habituation. However, in fasting condition, the concentrations were not affected, indicating that concentrations had returned to baseline levels after an overnight fast. This fact is consistent with the four hours half-life that has been reported for homocysteine in humans. High concentrations of postprandial homocysteine have been considered a risk factor for CVD.47
In a study with healthy individuals, it was verified that the consumption of high-protein diet, in relation to a hyperglycemic diet, results in increased concentrations of high-density lipoprotein-cholesterol (HDL-c), and no effects in the risk marker of CVD, such as reactive C protein9, were observed. Similarly, Farnsworth et al.48 observed a decrease in fatty acid concentrations, triglycerides, low-density lipoprotein-cholesterol (LDL-c) and increased HDL-c. Parker et al.49 verified reduced total cholesterol and LDL-c concentrations after individuals ate a high-protein diet rich in monounsaturated lipids.
On the other hand, according to review performed by Halton & Hu,19 there are evidences that high-protein ingestion leads to renal overcharge, which results in increased glomerular filtration and increased risk of kidney stone. However, this renal change has not been always identified. Gannon et al.6 evaluated the consumption effects of a diet with high content of protein (CHO: 40%, LIP: 30%, PTN: 30%) and other normoproteic (CHO: 55%, LIP: 30%, PTN: 15%), for five weeks, in non-treated diabetic individuals. In that study, it was verified a significant decrease in the triglyceride concentrations (20%) and total cholesterol, without any changes in the fractions of HDL-c and LDL-c. It was not verified change in the indicators of renal function (microalbumine and clearance of creatinine),6 either. However, these results must be carefully analyzed, since the patients were under medicine treatment for lipid control. Thus, long-term studies must be performed in order to investigate the effects of the consumption of a high-protein diet on the renal function.
Similar results were observed in another study, in which 18% versus 30% of proteins were ingested during nine weeks. In this study, it was not verified any change in the renal function. On the other hand, beneficial effects were observed related to weight reduction and body fat, which could be considered indicators of improvement in cardiovascular health.50 In another study, the consumption of a high-protein diet (27%), containing vegetable protein (wheat gluten), during a month, provided reduced oxidated LDL-c concentrations, triglycerides and uric acid, without affecting the release of creatinine.51
Meanwhile, according to Barzel & Massey20 and Massey,52 the excessive consumption of protein in such diet may cause hypercalciuria, which may affect bone health. To Massey,52 the effects of animal or vegetable protein on urinary calcium and bone metabolism may be influenced by other nutrients, such as soya isoflavones, vitamin D, caffeine and salt.
During twenty weeks, in a work with Sprague Dawley rats, the consumption of high-protein diets (6% of casein + 24% of a protein source) demonstrated that diets with soy and beef did not affect calciuria, in opposition to the diets with lactalbumin, egg, gelatin and casein. All diets presented equivalent quantities of magnesium, phosphor and calcium.53 According to the authors, high-protein diets increase the glomerular filtration, thus contributing to higher loss of calcium. In addition, the end products of amino acid catabolisms, such as sulfate, oxalate and sodium may hinder or compete with dietary calcium renal reabsorption, which promotes the loss of this macronutrient through the urine. A significant correlation was observed among these parameters (sulfate, oxalate and sodium) and the excretion of urinary calcium.53 However, according to Bell et al.,54 bone reabsorption does not seem to be affected by high protein consumption when the intake of calcium and phosphor is adapted. However, Allen & Hall55 declare that rats are not appropriate animals for this type of study, since they excrete a low percentage of calcium from the diet through the urine.
Notwithstanding, the evidences from studies with human beings are still insufficient to evaluate the possible renal changes connected to the high-protein diet intake, mainly in relation to protein of animal origin. Thus, further studies are necessary to find out the long-term effects of the increase of this macronutrient on daily nutrition, especially for susceptible groups, such as diabetics and individuals with organic disorders, mainly renal diseases.
Conclusion
Results of short-term studies have evidenced several beneficial effects of the intake of diets with high content of proteins (22 to 30% of daily calorie intake) from different sources (animal or vegetable) on glycemic control. However, long-term clinical tests are still necessary for further knowledge about the consequences of high protein consumption for the renal function, bone health and CVD development risks. The results of the mentioned works also suggest that the consumption of proteins of high biological amount, such as those of animal origin, mainly whey, which is rich in branchedchain amino acids, presents beneficial effects on glycemic homeostasis, resulting in increased insulin secretion and glucose gain by cells.
However, the results of the studies in which the effects of quantity and quality of proteins on glycemic response were evaluated are very controversial. The methodological problems they presented may be the cause of divergences. Thus, well-controlled studies are necessary, with the participation of individuals that have presented such diseases for a long time (for diabetics), with homogenous age and BMI, to avoid differences in secretion and/or insulin resistance that affect the glycemic response differently. Furthermore, the carbohydrate content in the tested diets will be maintained constant to prevent differences in the content of this macronutrient from affecting the blood glucose concentration and the results. The tested diets should not differ in consistency, since liquid diets do not require chewing and could present faster intestinal transit and absorption, resulting in more postprandial glycemic increase, in comparison to diets with solid consistency.
Therefore, we conclude that studies without the interference of confusing factors, such as those above mentioned, are necessary to identify the mechanisms and magnitude of protein effects on glycemic response, and identify the quantity and quality of this macronutrient to be ingested for the achievement of beneficial effects, without harm to health.
References
1. Wolever T, Jenkins DJA, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr 1991; 54: 846-54. [ Links ]
2. Pi-Sunyer FX. Glycemic index and disease. Am J Clin Nutr 2002; 76 (Suppl.): 290-8. [ Links ]
3. Baum JI, Layman DK, Freund GG, Rahn KA, Nakamura MT, Yedell BE. A reduced carbohydrate, increased protein diet stabilizes glycemic control and minimizes adipose tissue glucose disposal in rats. J Nutr 2006; 136: 1855-61. [ Links ]
4. Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet 2004; 364: 778-785. [ Links ]
5. Guttierres APM, Alfenas RCG. Efeitos do índice glicêmico no balanço energético. Arq Bras Endocrinol Metab 2007; 51 (3): 382-388. [ Links ]
6. Gannon MC, Nutall FQ, Saeed A, Jordan K, Hoover. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 2003; 78: 734-41. [ Links ]
7. Chacra AR. Efeito fisiológico das incretinas. Johns Hopkins Advanced Studies in Medicine 2006; 6 (7B): 613-17. [ Links ]
8. Johnston CS, Buller AJ. Vinegar and peanut products as complementary foods to reduce postprandial glycemia. American Diabetic Association 2005; 105 (12): 1939-42. [ Links ]
9. Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck I. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 2004; 80: 1246-53. [ Links ]
10. Calbet JA, MacLean DA. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr 2002; 132: 2174-82. [ Links ]
11. Brand-Miller J, Foster-Powell K. Diets with a low glycemic index: from theory to practice. Nutrition Today 1999; 34 (2): 64-72. [ Links ]
12. Van loon LJC, Saris WHM, Verhagen H, Wagenmakers AJM. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr 2000; 72: 96-105. [ Links ]
13. Institute of Medicine and Food & Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington, DC: National Academy Press; 2005. [ Links ]
14. Weigle DS, Breen PA, Mattys CC, Callahan HS, MeeuWs KE, Burden VR et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weigh despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005; 82: 41-8. [ Links ]
15. Westphal SA, Gannon MC, Nuttall FQ. Metabolic response to glucose ingested with various amounts of protein. Am J Clin Nutr 1990; 52: 267-72. [ Links ]
16. Blaak EE. Prevencion and treatment of obesity and related complication: a role for protein? Inter J Obes 2006; 30: 24-7. [ Links ]
17. Nutall FQ, Mooradian AD, Gannon MC, Billington CJ, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984; 7: 465-70. [ Links ]
18. Verhoef P, Vliet TV, Olthof MR, Katan MB. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am J Clin Nutr 2005; 82: 553-8. [ Links ]
19. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weigh loss: A critical review. J Am Coll Nutr 2004; 23 (5): 373-85. [ Links ]
20. Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr 1998; 128: 1051-3. [ Links ]
21. Sgarbieri VC. Propriedades fisiológicas-funcionais das proteínas do soro do leite. Rev Nutr 2004; 17 (4): 397-409. [ Links ]
22. Moghaddam E, Vogt JA, Wolever TMS. The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr 2006; 136: 2506-11. [ Links ]
23. Mourão DM, Bressan J, Campbell WW, Mattes RD. Effects of food formo n appetite and energy intake in lean and obese young adults. Inter J Obes 2007; 1-8. [ Links ]
24. Cisternas JR. Fisiologia das ilhotas de Langerhans. In: Douglas CR. Tratado de fisiologia aplicado à nutrição. Robe: São Paulo 2002. 1046 p. [ Links ]
25. Manders RJ, Koopman R, Sluijsmans WE, Van den Berg R, Verbeek, Saris WH, et al. Co-ingestion of a protein hydrolysate with or without additional leucine effectively reduces postprandial blood glucose excursions in type 2 diabetic men. J Nutr 2006; 136: 1294-9. [ Links ]
26. Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev 2010; 68 (5): 270-9. [ Links ]
27. Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr 2003; 133: 261S-7S. [ Links ]
28. Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary Leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanism. Diabetes 2007; 56: 1647-54. [ Links ]
29. Frid AH, Nilsson M, Holst JJ, Bjorck. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr 2005; 82: 69-75. [ Links ]
30. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104 (6): 787-94. [ Links ]
31. Bahia L, Gomes BM. Influência da idade e do diabetes sobre esteróides sexuais e SHBG em homens. Arq Bras Endocrinol Metab 2003; 47 (3): 256-60. [ Links ]
32. Dantas JR, Almeida MH, Barone B, Campos F, Kupper R, Milech A, Zajdenverg L, Rodacki M, Oliveira JEP. Avaliação da função pancreática em pacientes com diabetes melito tipo 1 de acordo com a duração da doença. Arq Bras Endocrinol Metab 2009; 53 (1): 64-71. [ Links ]
33. Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 2010; 91: 966-75. [ Links ]
34. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci 1997; 94: 14930-5. [ Links ]
35. De Graaf C, Blom WAM, Smeets PAM, Stafleu A, Hendriks HFJ. Biomarkers of satiation and satiety. Am J Clin Nutr 2004; 79: 946-61. [ Links ]
36. Nordt TK, Besenthal I, Eiggstein M, Jakober B. Influence of breakfasts with different nutrient contents on glucose, C peptide, insulin, glucagon, triglycerides, and GIP in non-insulin-dependent diabetics. Am J Clin Nutr 1991; 53: 155-60. [ Links ]
37. Collier G, O' Dea K. The effect of coingestion of fat on the glucose, insulin, and gastric inhibitory polipeptide responses to carbohydrate and protein. Am J Clin Nutr 1983; 37: 941-4. [ Links ]
38. Baer DJ, Rumpler WV, Miles CW, Fahey GC. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr 1997; 127: 579-86. [ Links ]
39. Ellis PR, Kendall CW, Ren Y, Parker C, Pacy JF, Waldron KW, Jenkins DJA. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr 2004; 80: 604-613. [ Links ]
40. Traoret CJ, Lokko P, Cruz ACRF, Oliveira CG, Costa NMB, Bressan J et al. Peanut digestion and energy balance. Int J Obes 2008; 32: 322-8. [ Links ]
41. Brito CJ, Volp ACP. Suplementação de arginina no exercício. Nutrição Brasil 2007; 6: 306-11. [ Links ]
42. Gannon MC, Nutall JA, Nutall FQ. Oral arginine does not stimulate an increase in insulin concentration but delays glucose disposal. Am J Clin Nutr 2002; 76: 1016-22. [ Links ]
43. Bao J, Jong V, Atkinson F, Petocz P, Brand-Miller JC. Food insulin index: physiologic basis for predicting insulin demand evoked by composite meals. Am J Clin Nutr 2009; 90: 986-92. [ Links ]
44. Paiva AC, Alfenas RCG, Bressan J. Efeitos da alta ingestão diária de proteínas no metabolismo. Rev Bras Nutr Clin 2007; 22 (1): 83-8. [ Links ]
45. Schuette AS, Zemel MB, Linkswiler HM. Studies on the mechanism of protein induced hypercalciuria in older men and women. J Nutr 1980; 110: 305-15. [ Links ]
46. Johnston CS, Tjonn SL, Swan PD, White A, Hutchins H, Sears B. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am J Clin Nutr 2006; 83: 1055-61. [ Links ]
47. Graham IH, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA 1997; 277: 1775-81. [ Links ]
48. Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of high-protein, energy-restricted control, and lipid concentration in overweight and hyperinsulinemic men an women. Am J Clin Nutr2003; 78: 31-9. [ Links ]
49. Parker B, Noakes M, Luscombe N, Clifton P. Effect of a hight-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425-430. [ Links ]
50. Leidy HJ, Mattes RD, Campbell WW. Effects of acute and chronic protein intake on metabolism, appetite, and ghrelin during weight loss. Obesity 2007; 15 (5): 1215-25. [ Links ]
51. Jenkins DJA, Kendall CWC, Vidgen E, Augustin LSA, Van Erk M, Geelen A et al. High-Protein diets in hiperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. Am J Clin Nutr 2001; 74: 57-63. [ Links ]
52. Massey LK. Dietary animal and plant protein and human bone health: a whole foods approach. J Nutr 2003; 133: 862S-5S. [ Links ]
53. Calvo MS, Bell RR, Forbes RM. Effect of protein-induced calciuria on calcium metabolism and bone status in adult rats. J Nutr 1982; 112: 1401-13. [ Links ]
54. Bell RR, Engelmann DT, Sie TL, Draper HH. Effect of a high protein intake on calcium metabolism in the rat. J Nutr 1975; 105: 475-83. [ Links ]
55. Allen LH, Hall TE. Calcium metabolism, intestinal calcium-binding protein, and bone growth of rats fed high protein diets. J Nutr 1978; 108: 967-72. [ Links ]
![]() Correspondence:
Correspondence:
Fernanda Cristina Esteves de Oliveira.
Universidade Federal de Viçosa.
Departamento de Nutrição e Saúde.
Avda. P. H. Rolfs, s/n.
36570-000 Viçosa. Minas Gerais. Brazil.
E-mail: fernandaesteveshad@yahoo.com.br
Recibido: 22-III-2010.
1a Revisión: 4-X-2010
2a Revisión: 19-X-2010
Aceptado: 16-XI-2010.