Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.6 Madrid nov./dic. 2013
https://dx.doi.org/10.3305/nh.2013.28.6.6892
REVISIÓN
Systematic review; nutritional therapy in gestational diabetes mellitus
Revisión sistemática; terapia nutricional en la diabetes mellitus gestacional
Helaine Thomaz de Lima1, Eliane Lopes Rosado2, Paulo Augusto Ribeiro Neves1, Raphaela Corrêa Monteiro Machado1,3, Larissa Mello de Oliveira1,3 y Cláudia Saunders1
1Research Group on Maternal and Child Health (Grupo de Pesquisa em Saúde Materna e Infantil - GPSMI). Instituto de Nutrição Josué de Castro/Universidade Federal do Rio de Janeiro (UFRJ).
2Instituto de Nutrição Josué de Castro/UFRJ.
3Maternidade Escola/UFRJ. GPSMI. Instituto de Nutrição Josué de Castro/UFRJ. Brazil.
ABSTRACT
Introduction: Several methods of dietetic counseling can be used in the nutritional therapy in gestational diabetes mellitus (GDM). The main methods are the traditional method (TM) and the carbohydrate counting (CCM).
Objective: Presenting a systematic review of the literature on the impact of nutritional therapy in GDM, through TM and CCM, evaluating the results for maternal and child health.
Methods: We searched databases PubMed, Scopus, Web of Science, Lilacs and CAPES Digital Bank of Thesis. The methodological quality of all the studies included was made using the Jadad score.
Results and conclusion: We have found five studies that evaluated the effects of nutritional therapy, through the TM, on the maternal and child health. None study evaluating the CCM was detected in pregnant women with GDM Nutritional therapy given during antenatal care was effective in reducing pregnancy complications (preeclampsia, excessive gestational weight gain, necessity for cesarean delivery, for insulin therapy and for shoulder dystocia), perinatal complications (macrosomia, neonatal hypoglycemia, and birth weight) and also in better glycemic control. The use of nutritional therapy should be highlighted within the antenatal care for pregnant women with GDM, giving the satisfactory results on metabolic control and on pregnancy outcomes. Studies examining the CCM to GDM patients should be conducted to show its effects on maternal and child health.
Key words: Gestational diabetes mellitus. Nutrition therapy. Glycemia. Pregnancy. Prenatal care.
RESUMEN
Introducción: Diversos métodos de asesoramiento dietético pueden ser utilizados en la terapia de la nutrición en la diabetes mellitus gestacional (DMG). Los principales son el método tradicional (MT) y el contaje de hidratos de carbono (MCHC).
Objetivo: Presentar una revisión sistemática de la literatura sobre el impacto de la terapia nutricional en el DMG, utilizando el MT y MCHC, la evaluación de los resultados para la salud materna e infantil. Métodos: Se realizó una búsqueda electrónica a través de las siguientes bases de datos: PubMed, Scopus, Web of Science, Lilacs y CAPES Banco Digital de Tesis. La calidad metodológica de todos los estudios incluidos se evaluó mediante la escala de Jadad.
Resultados y Conclusiones: Se encontraron cinco estudios que evaluaron los efectos de la terapia nutricional utilizando el método tradicional, en la salud de la mujer embarazada y su feto. No se detectó ningún estudio que tenga evaluado el MCHC en las mujeres embarazadas con DMG. La terapia nutricional durante la atención prenatal fue eficaz em la reducción de las complicaciones del embarazo (pre-eclampsia, aumento excesivo de peso, necesidad de parto por cesárea, terapia con insulina y distocia de hombros), las complicaciones perinatales (macrosomía, hipoglucemia neo natal, peso al nacimiento) y también en un mejor control glucémico. El uso de la terapia nutricional debe ser destacada en la atención prenatal para las mujeres embarazadas con DMG, dados los resultados satisfactorios en el control metabólico y complicaciones en el embarazo. Los estudios que evalúan el MCHC en las mujeres embarazadas con DMG deben llevarse a cabo para mostrar sus efectos en la salud materna e infantil.
Palabras clave: Diabetes mellitus gestacional. Terapia nutricional. Glucemia. Embarazo. Atención prenatal.
Abbreviation list
GDM : Gestational diabetes mellitus.
ADA: American Diabetes Association.
TM: Traditional method.
CCM: Carbohydrate counting method.
A1C: Glycated hemoglobin.
US: United States.
IG: Intervention group.
CG: Control group.
OGTT: Oral glucose tolerance test.
RR: Relative risk.
SD: Standard deviation.
BMI: Body mass index.
GWG: Gestational weight gain.
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or initial recognition during pregnancy1. Approximately 7% of pregnancies are complicated by GDM2.
The Ministry of Health of Brazil3 recognizes that GDM therapy should be based on a healthy diet, exercising and glycemic control, with or without insulin. The nutritional therapy is considered essential in the treatment of GDM, since it helps to avoid excessive gestational weight gain, minimizing the occurrence of macrosomic fetuses and neonatal complications4,5. The American Diabetes Association (ADA)1 and the Ministry of Health of Brazil3 claim that GDM patients could achieve metabolic control only with nutritional therapy and lifestyle changes.
Various methods of dietary counseling may be used in the nutritional therapy in diabetes mellitus, including GDM6-8. Among them are: the glycemic index that is based, mainly, on replacing higher glycemic index foods by lower ones throughout the day6; the method based on the energy distribution of macronutrients in meals - called by the authors of this article as the traditional method (TM)- which is a proposal of energy distribution of macronutrients, and may vary with each meal, suggesting a smaller proportion of energy in small meals7; and the carbohydrate counting method (CCM)8, which it is important to consider the total of carbohydrate consumed per meal, being that the amount of carbohydrate has a higher priority than its type or source9.
The proportion of macronutrients recommended for GDM patients have not been established in a consensus and there are still differences between national and international recommendations3,10-12.
Ideally, all GDM patients should receive dietary advice by dietitians throughout gestation4,5. The accompaniment of the nutrition during pregnancy aims to obtain adequacy of dietary advice, and may be used by the three methods described above for nutritional therapy. However, it should be noted that the results obtained clinically using the glycemic index as nutritional therapy in GDM, are still controversial13,14, therefore the proposal of ADA is to use the TM and the CCM, preferably, for GDM11.
The evidences about the benefits of using the different methods of nutritional therapy on obstetric outcomes and glycemic control in GDM are not clearly defined yet. Studies analyzing such proposals of treatment did not reach an agreement on what would be the most effective method, besides the fact that there are few studies that have evaluated this approach15-20.
Studies show benefits for both mother and child with the application of TM in GDM. The use of this method, through individual consultations with a nutritionist, has demonstrated significant reduction 1-4% in perinatal complications21, in the need of insulinization15, in the prevalence of larger neonates for gestational age16.
Regarding the CCM, for type 1 diabetes mellitus individuals, some studies demonstrated substantial improvement in glycemic control without weight gain16, as well as a reduction in the concentration of glycated hemoglobin (A1C), in episodes of severe hypoglycemia, in faster insulin doses, without the existence of increased body weight22, because it allowed greater flexibility in the choice of food and the satisfaction of individuals23. Studies about application and efficacy of the method for GDM patients are unknown.
The objective of this article was to conduct a systematic review of controlled clinical trials on the evidence on the impact of nutritional therapy, based on TM and CCM, in GDM patients on the occurrence of pregnancy complications and perinatal outcomes, as well as in glycemic control and insulin need.
Methods
The search for articles came from extensive research in the following databases: PubMed, Scopus, Web of Science, Lilacs and Bank CAPES Thesis. This search occurred between the months of November and December 2012. All procedures used in this systematic review have followed the recommendations of PRISMA Statement24.
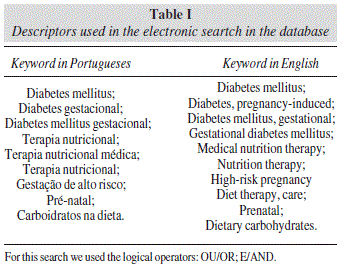
The keywords used for the literature search are summarized in table I. These were determined using the terms in the DeCS (Descritores em Saúde) for terms in Portuguese and MeSH (Medical Subject Headings) for English. Articles that would be included in the study should be in the following languages: Portuguese, English and Spanish.
For the selection of studies, inclusion criteria were adopted such as: controlled clinical trial, originals, where the participants should be adult women diagnosed with GDM; use of nutritional therapy for GDM by TM and CCM; studies which were approved by the Ethics Committee in Research. Exclusion criteria were: studies conducted with adolescent pregnant; with ones who had more than one fetus; work on animals; presence of diabetes mellitus type 1 or 2 prior to pregnancy; presence of previous diseases to pregnancy requiring dietary treatment; studies that used other types of treatment for GDM combined with nutrition therapy, besides the use of insulin; studies using any other type of nutritional therapy for the GDM, in addition or not to therapies described above. It was not delineated in this review since what year of publication the papers would be included.
The search for studies was performed in duplicate, where the authors HTL and PARN made the selection of the ones to be included. The pursuit process for article was started from reading the titles. After this initial stage, the selected papers were reviewed by reading their abstracts available. Then, these selected studies were separated for further analysis to identify relevant publications, according to the inclusion/exclusion criteria. As a complement to the search of scientific evidences, the reference lists of each article included in the review were consulted for the identification of probable important studies that had not been found previously.
Although we included only the clinic trials, a search in the Cochrane database was also conducted in order to find systematic reviews and meta-analyzes about the topic. These reviews found were not included in this systematic analysis, but may be useful to identify other articles through the reading of references lists, which could be included in this study.
We considered as significant results in each study, those which presented a p-value < 0.05 and/or measures of association with confidence intervals (95% or 97%) that did not correspond to the value 1. We assessed the methodological quality of all included studies using the Jadad25 scale, which analyzes the quality of clinical trials, based on information about the issue of randomization, the study "double-blind" and the comments needed on the possible samples loss along the research. This score ranges from 0 to 5 points, considering high-quality studies those with scores greater than 3.
Results
Were initially identified 53 publications. After reading the list of references found as base of Cochrane studies, we found two other articles, and thus, reaching a total of 55 studies. After the first evaluation, through titles and abstracts, we excluded 36 articles, resulting in 19 publications. These remaining studies were entirely read so we could coming to an end with 5 articles. Details of the studies search are described as a flowchart in figure 1.
No studies that used de CCM for the treatment of GDM patients were found. Thus, the included randomized trials had the purpose of evaluating the role of nutritional therapy by the TM, aiming at GDM on obstetric and perinatal outcomes, in metabolic control diabetes, among others. The countries in which trials occurred were the United States (US), Canada, Australia and Poland.
All participants were adults with the diagnosis of GDM in the second trimester. All included articles are in english. It was not possible to perform a meta-analysis due to the fact that the studies found had different characteristics, such as different methods to diagnose GDM.
In table II are summarized the characteristics of the articles included in relation to where the work was conducted, the time when the intervention began, methods used for diagnosis, the number of participants in each study, the main results and the score obtained by the used scale.
The studies that presented better methodological quality, in descending order, were Garner et al.26, Crowther et al.21, Landon et al.16, Reader et al.15 and Cypryk et al.27. The first two managed a score of 5 on the Jadad25 scale, since they described all necessary points and informed about the use of an adequate method to randomization.
Some common features of all the publications included are: they have an intervention group (IG), which received a nutritional therapy according to the objective of each study, and a control group (CG), which received routine prenatal care of each studied unit (except for Cypryk et al.27); women of all CG were instructed to follow a healthy diet during pregnancy, but did not receive nutritional counseling; the need for glucose monitoring of the pregnant women and laboratory tests for GDM control.
Garner et al.26 evaluated which model for glycemic control has greater impact on reducing macrosomia, birth trauma, neonatal hypoglycemia and in the occurrence of surgical delivery. The IG was monitored by an obstetrician and an endocrinologist, in Ottawa, Canada. This group received nutritional therapy from a restricted diet of 35 kcal/kg of ideal body weight/day.
The average weight gain of IG and CG was of 13.3 kg and 12.5 kg, respectively, with no significant difference. There were no significant differences in the average fasting glucose test and in the oral glucose tolerance test (OGTT) in the beginning of the study. Within the IG group, 24.2% of the women required insulin therapy. After two weeks of study, there was a significant difference in the average of fasting glucose between groups, it was lower in IG (p = 0.0006), but not at the OGTT 1h. In the 30-32 weeks of gestation, the IG group had lower fasting glucose concentrations than the CG (80.3 mg/dL; standard deviation (SD) = 14.76 and 84.6 mg/dL; SD = 18.8; respectively; p = 0.035) and also the OGTT 1h (126.18 mg/ dL; SD = 25.2 and 135.36 mg/dL; SD = 34.14, respectively; p = 0.009). There were no differences concerning the frequency of neonatal hypoglycemia, hyperbilirubinemia, as well as in types of delivery (vaginal or cesarean). There were also no differences between birth weight (p = 0.118) and the occurrence of macrosomia26.
The main objectives of Crowther et al.21 study were to evaluate the nutritional treatment proposed by national Australian health department on perinatal complications and obstetric outcome. Women with risk factors to GDM or with alterations in OGTT with 50 and 75g of dextrose were considered eligible, and a new test was performed to identify pregnant women with glucose intolerance. Those that showed positive screening for GDM were part of the IG. Women with altered concentrations of glucose were given insulin.
In the beginning of study, 93% of the subjects were considered at risk for GDM, according to the OGTT. The occurrences of serious perinatal problems (perinatal death, shoulder dystocia, bone fracture, and nerve palsy) were significantly lower in IG than in CG (p = 0.01 -adjusted for maternal age, ethnicity/race and parity). Levels of statistical significance were not found among the groups in need of phototherapy and cesarean section. No perinatal deaths occurred in IG, but 5 were registered in CG (one of them associated with preeclampsia and intrauterine growth restriction). The children of the CG were smaller in comparison to the IG ones (p < 0.001) and they were also premature. There were fewer cases of fetal macrosomia in the IG than in the CG (p < 0.001). The IG women received more visits from health professionals involved in the care of GDM (p < 0.001) and presented less gestational weight gain (p = 0.01). Most IG women used insulin (20% in IG and 3% in controls)21.
In US, Landon et al.16 had the objective of determining if a treatment proposal reduces the perinatal and obstetric complications. Women initially considered eligible for the study (fasting glucose above 135 and under 200 mg/dL) were submitted to a new fasting glucose test and also to an OGTT. Those who presented an inadequate glucose level in the second test were diagnosed with GDM. The complications were divided into perinatal (primary and secondary) and maternal.
No statistically significant differences were found regarding the socio-demographic characteristics, the initial OGTT among the study groups, frequency of primary perinatal problems (gestational age at birth, hypoglycemia, hyperbilirubinemia, elevated concentrations of C-peptide in cord, perinatal death and birth trauma), being 32.4% in IG and 37% in CG (relative risk - RR 0.87 [97% CI = 0.72-1.07]; p = 0.14), even after adjusting the alcohol consumption during pregnancy. Differences between IG and CG appeared respectively in secondary perinatal alterations: birth weight (3302 g - SD 502.4; 3408 g - SD 589.4; p < 0.001), macrosomia (RR 0.41 [97% CI = 0.26-0.66]; p < 0.001), large for gestational age (RR [97% CI 0.32-0.76], p < 0.001) and adipose tissue (427.0 g - SD = 197.9, 464.3 g - SD = 222.3; p = 0.003). No differences were found among small for gestational age (p = 0.49) and neonatal intensive care unit admission (p = 0.19)16.
The differences found among IG and CG related to maternal characteristics were in cesarean section (RR 0.79 [97% CI 0.64-0.99]; p = 0.02), shoulder dystocia (RR 0.37 [97% CI 0.14-0.97]; p = 0.02), preeclampsia (RR 0.46 [97% CI 0.22-0.97] p = 0.02), BMI at birth (31.3 kg/m2 - 5.2 SD, 32.3 kg/m2 - SD 5.2, p < 0.001) and total gestational weight gain (2.8 kg - SD 4.5, 5.0 kg - SD 3.3, p < 0.001). The average number of prenatal visits was higher in IG (n = 7) than in CG (n=5) (p < 0.001)16.
Reader et al.15 assessed if the nutritional care taken by nutritionists using specific US guidelines for GDM, results in a different and improved obstetric outcome. This was conducted in 20 US states, in registered clinics (obstetrics, endocrinology). The 25 clinics that participated in the study were randomly divided into IG (n = 12) and control group (n = 13). The IG participants should have at least 3 consultations with nutritionists. There are no evaluations about the resemblance in sociodemographic characteristics of the participants in Reader et al.15 study.
Regarding the use of insulin, it was lower in IG when we compare it with CG (24.6% and 31.7%, respectively, p = 0.05) and it happened earlier in CG, nevertheless the numbers found are not statistically significant (p = 0.075). Among the factors associated with insulin use are A1C base, height, pre-pregnancy BMI, gestational age at diagnosis of GDM and duration of nutritional care. The proportion of women with high A1C in the beginning did not differ between groups (6.1% IG, 8.6% CG, p = 0.58) and did not differ at the end, but the percentage difference of A1C between one group and the other increased (7, 1% IG, 13.8% CG, p = 0.25). The groups also did not differ with respect to cesarean section (p = 0.67), macrosomia (p = 0.98), low weight at birth (p = 0.27) and prematurity (p = 0.25), but the CG had more than twice as premature (10.6% CG, 4.6% IG) babies than the IG. The babies' length at birth and the Apgar score 1 'and 5' did not differ between groups. Prenatal care was higher among women who required insulin compared to those who did not use the same treatment, in an analysis of combined data (p = 0.07)15.
Evaluate the effectiveness and safety of low and high carbohydrate diets, as well as its impact on blood concentrations of glucose and urinary ketones, was the goal of Cypryk et al.27, in Poland. The GDM patients were separated in groups: one would receive a diet with 45% of total energy intake from carbohydrates (GA) and another would receive a diet with 65% carbohydrates (GB). Regarding the other macronutrients, in GA 25% of the energy came from protein and 30% from lipids; in GB it was offered 25% protein and 15% fat. The program took place for only 14 days. Fasting glucose tests and monitoring during the day were performed along the study27.
The average fasting blood glucose levels did not differ significantly between the two groups before the beginning of the treatment (p > 0.05). In GA no differences were found between the fasting glucose before and after treatment (p = 0.414), but there were differences between the glucose levels after breakfast (p = 0.021), after lunch (p = 0.023) and after dinner (p = 0.011). In GB was found an association between glucose after lunch (p = 0.012) and after dinner (p = 0.003), before and after treatment. There was no occurrence of ketonuria, but it was necessary to use insulin in two pregnant women from GA and one from GB. No significant differences were found regarding the gestational age at delivery, type of delivery (vaginal or cesarean), prenatal care, macrosomia and Apgar score (p > 0.05)27.
Discussion
Few randomized controlled trials addressing the use of nutritional therapy in the treatment of GDM were found. It is important to emphasize that none of these were conducted in Brazil. Another important point to be emphasized is about the methodological differences in each study, and also the limited number of studies that makes it difficult to extrapolate the results observed.
Despite the existence of national and international5,8 guidelines recommending the use of CCM as nutritional therapy strategy, we did not find any publication with GDM patients. Several researches17,22,23 using this method for the treatment of type 1 diabetes, elucidated satisfactory results in controlling the disease, highlighting the importance that such proposal should be evaluated in the GDM.
None of the articles in this review had similarity about the cutoff points for diagnosis of GDM. Due to this, the research is impaired by the multiplicity of diagnostic criteria and the lack of standards of the cutoff points to identify GDM. The use of different methods for diagnosing a disease may cause an over or underestimation of it. Early diagnosis of GDM not only aims to minimize the adverse maternal-fetal effects, but also to identify women at increased risk for developing type 2 diabetes mellitus), helping the entire process of therapeutic monitoring of these women28. The ADA1 recommends as screening method of diagnosis the simplified OGTT, overloaded with 50 g in all pregnant women between 24 and 28 weeks of pregnancy. This is commonly the most used screening method, recognized as the "gold standard", considering as cutoff point the glucose of 1 hour after the overload equal to or over 140 mg/dL.
Nevertheless, it could be observed that all studies showed satisfactory results for the use of nutritional therapy for GDM, which brought benefits to the mother and fetus. However, it is important to highlight that some methodological issues noted in those studies object the methods used for the intervention.
In Garner et al.26 research, the IG received nutritional therapy with calorie-restricted diet containing 35 kcal/kg of ideal body weight/day and with the daily fragmentation of the meal, without any evaluation of the nutritional status before pregnancy. Despite the satisfactory results, the nutritional recommendations should be individualized, based on the classification of pre-pregnancy BMI and the definition of total and weekly gestational weight gain (GWG)29-31.
Padilha et al.32 tested the impact of nutritional intervention by nutritionist in nondiabetic women, in which the diet was individualized based on detailed nutritional assessment, including anthropometric measurements, showing the positive effects of this individualized intervention in the adequacy of the total gestational weight gain. These recommendations should be tested during prenatal care of pregnant women with GDM, considering that the adequacy of GWG may be related to better perinatal outcomes33 and that the recommendation of gestational weight gain is the same for healthy women and those with GDM.
Crowther et al.21 study point out that the different treatment for women with GDM reduces the perinatal morbidity and improves quality of life of both mother and child. The group that received nutritional intervention had a greater number of visits from health professionals and had lower GWG. The authors also describe that the nutritional care was individualized and the pre-pregnancy weight, activity level, dietary intake, and GWG were considered. But there was no methodological detail regarding the classification of nutritional status, percentage of macronutrients provided, level of physical activity prescribed and GWG recommendation21.
Landon et al.16 results demonstrate that nutritional therapy for GDM minimizes perinatal and obstetric complications, improving the quality of life of both mother and child, however it was not able to reduce maternal-fetal mortality. In this study there wasn't any specification of the nutritional therapy employed, leaving doubts about the used standards, since it is not clear if any specific nutritional recommendation for GMD was applied.
Reader et al.15 described advantageous effects to both mother and child with the monitoring during the prenatal of GDM patients. This multicenter study showed the difference regarding the dietary advice in several clinics in the US, validated for the GDM treatment, where the survey was conducted, thus obtaining satisfactory obstetric outcomes. The authors state that there are still many unanswered questions about the nutritional therapy for GDM, for instance, the changes in carbohydrate diet, energy needs and specific GWG for GDM, thereby pointing out the necessity of more studies that seek such answers.
Also in relation to the study presented, the authors describe that the frequency and duration are factors that must not be forgotten during nutritional consultation and they are as important as nutritional advice. They recommend that pregnant women receive nutritional counseling in 48 hours after the diagnosis and a minimum of three nutritional consultations15. Other publications, but with pregnant women without GDM, demonstrate the benefits of nutritional care during prenatal both for the mother and the fetus, starting in the 1st trimester31-37.
The mentioned authors15,16,21,26,27 emphasize that individualized nutritional care for women with GDM provides better perinatal outcomes. However within the methods of these studies there are no explanation concerning some topics that deserve attention, such as the participation in the proposed feed plan, the principles of quantity, quality (macronutrient %), meals pattern; adequacy to the GWG; detailed anthropometric assessment (except for Crowther et al.21, but they left a gap on how he assessed the pregestational nutritional status of these pregnant women).
At Cypryk et al.27 work the ways of anthropometric calculation and GWG are not detailed. It is reported that all pregnant women received about 1800 Kcal/day, but there was an emphasis on diet individualization. They also describe that there was a daily distribution of macronutrients, but they did not demonstrate an energy distribution in the daily meals, as described by ADA7.
These issues are of great importance and fundamental for nutritional therapy, with the goal of reaching a recommended weight gain, normoglycemia and contribute to a healthy lifestyle, even after the end of pregnancy31. However, the ADA11 points out that the amount of carbohydrates in the diet should not be less than 175 g/day in order to improve blood glucose and minimize the risk of ketonemia or ketoacidosis, which effectively brings undesirable obstetric results.
The individualization of nutritional care for pregnant women should be reinforced and practiced, because this way it is possible that the nutritional guidance is planned according to the individual characteristics of each individual, within the environment in which each woman lives31,32,36.
The lack of studies conducted with Brazilian women, based on CCM generates a gap about the possible benefits and harms of this intervention in this population.
The scientific literature describes the need for follow-up with professional nutritionists during prenatal of GDM patients33, but there is not a recommendation or consensus on the number of nutrition queries that would be sufficient to minimize the risks of perinatal in women with GDM.
Brazil's Ministry of Health3 reports that in pregnancies considered at risk, such as the case of GDM, there should be a higher frequency of home visits and consultations, and the interval defined in accordance to the identified risk factor and the condition of the mother at the time.
There is still controversy about nutritional therapy in GDM on the GWG, energy recommendations, distribution and food composition (amount and types of fat and carbohydrates, as well as its restriction)2,3, thus the proper daily schedule of quantity and size of meals is essential. The dietary guidance is able to provide a good control of fasting and postprandial glucose.
In short, we can say that the great advantages of the use of nutritional therapy in the treatment brings to GDM patients the reduction of maternal complications (preeclampsia, GWG excessive, need for cesarean delivery, insulin therapy, improved metabolic control, shoulder dystocia) and perinatal complications (macrosomia, neonatal hypoglycemia, birth weight), showing the importance of professionals related to nutritional care during pregnancies considered of low or high risk.
Despite all these beneficial effects associated with nutritional care, there are still many questions about the real benefits of this practice. The studies involved in this review are of great importance, since most of them present important methodological advisement that allows demonstrating the credibility of the results found. However, the similarities between them are quite minimum and the benefits were not the same for all articles.
This shows how important it is to make further studies with larger sampling rate and with a standard diagnostic method. Studies conducted with the population of pregnant Brazilian with GDM are also needed, because the results of the research assessed are for populations of developed countries and this may differ in some way for populations of undeveloped countries. Analyzing the effects of other therapies in GDM should also be encouraged, especially the CCM, since the observed results of this method for type 1 and 2 diabetes mellitus reinforce that other therapeutic practices can be applied to the treatment of diabetes mellitus, which should possibly be extended for GDM.
Conclusion
The nutritional attention focused on the GDM is a crucial tool, as seen by the beneficial effects for maternal and child health and should be universalized and extended to all women, preferably running currently with early prenatal care. But still we haven't reached a consensus on the best method to be used as nutritional therapy on the specific needs of GDM women, especially since there is a large gap on the effects of CCM compared to TM, which is currently the most used in the GDM.
Finally, more studies are needed for further clarification regarding the use of nutritional therapy through other methods aiming at the diabetes to provide important information about the relevance of this intervention in our population of women with GDM.
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35 (Sup.1): S11-61. [ Links ]
2. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004; 27 (Sup.1): S88-90. [ Links ]
3. Ministerio da Saúde. Gestação de alto risco: Manual técnico. Brasília: MS; 2010. [ Links ]
4. Metzger BD, Buchanan TA, Coustan DR, Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop- Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30 (Supl. 2): S251-60. [ Links ]
5. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2007; 30 (Supl. 1): S4-41. [ Links ]
6. Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981; 34: 362-6. [ Links ]
7. American Diabetes Association. Position Statement: Nutrition recommendations and principles for people with diabetes mellitus. J Am Diet Assoc 1994; 94: 504 . [ Links ]
8. Sociedade Brasileira de Diabetes. Manual oficial de contagem de carboidratos para profissionais da saúde. Rio de Janeiro: SBD; 2009. [ Links ]
9. Wheeler ML, Daly A, Evert A, Franz MJ, Geil P, Holzmeister LA, et al. Choose your foods: Exchange lists for diabetes, Sixth edition, 2008: Description and guidelines for use. J Am Diet Assoc 2008; 108 (5): 883-8. [ Links ]
10. Institute of Medicine. Dietary reference intakes: Energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington DC: IOM, 2002. [ Links ]
11. American Diabetes Association. Nutrition recommendations and interventions for diabetes. A position statement of the American Diabetes Association. Diabetes Care 2008; 31 (Supl. 1): S61-78. [ Links ]
12. Philippi ST, Laterza AR, Cruz ATR, Ribeiro LC. Pirâmide alimentar adaptada: guia para escolha dos alimentos. Rev Nutr 1999; 12 (1): 65-80. [ Links ]
13. Scholl TO, Chen X, Khoo CS, Lenders C. The dietary glycemic index during pregnancy: Influence on infant birth weight fetal growth, and biomarkers of carbohydrate metabolism. Am J Epidemiol 2004; 159 (5): 467-74. [ Links ]
14. Louie JCY, Brand-Miller JC, Markocic TP, Ross GP, Moses RG. Glycemic index and pregnancy: A systematic literature review. J Nutr Metab 2010. doi: 10.1155/2010/282464. [ Links ]
15. Reader D, Splett P, Gunderson EP. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dietitians on pregnancy outcomes. J Am Diet Assoc 2006; 106: 1426-33. [ Links ]
16. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339-48. [ Links ]
17. Dias VM, Pandini JA, Nunes RR, Sperandei SL, Portella ES, Cobas RA, et al. Effect of the carbohydrate counting method on glycemic control in patients with type 1 diabetes. Diabetol Metab Syndr 2010; 2 (54): 1-7. [ Links ]
18. Louie JC, Markovic TP, Perera N, Foote D, Petocz P, Ross GP, et al. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011; 34 (11): 2341-6. [ Links ]
19. Balas-Nakash M, Rodríguez-Cano A, Muñoz-Manrique C, Vásquez-Peña P, Perichart-Perera O. Adherence to a medical nutrition therapy program in pregnant women with diabetes, measured by three methods, and its association with glycemic control. Rev Invest Clin 2010; 62 (3): 235-43. [ Links ]
20. Ma WJ, Qi BH, Zhang YJ, Huang ZH, Xiao BX, Li YH, et al. Application of different nutrition therapies in pregnancy with abnormal glucose metabolism. Zhonghua Yu Fang Yi Xue Za Zhi 2011; 45 (5): 426-9. [ Links ]
21. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352 (24): 2477-86. [ Links ]
22. Scavone G, Manto A, Pitocco D, Gagliardi L, Caputo S, Mancini L, et al. Effect of carbohydrate counting and medical nutritional therapy on glycaemic control in type 1 diabetic subjects: a pilot study. Diabet Med 2009; 27 (4): 477-9. [ Links ]
23. Hissa ASR, Albuquerque LL, Hissa MN. Avaliação do grau de satisfação da contagem de carboidratos em diabetes mellitus tipo 1. Arq Bras Endocrinol Metabol 2004; 48 (3): 394-7. [ Links ]
24. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009; 151: 264-9. [ Links ]
25. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials 1996; 17: 1-12. [ Links ]
26. Garner P, Okun N, Keely E, Wells G, Perkins S, Sylvain J, et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: A pilot study. Am J Obstet Gynecol 1997; 177: 190-5. [ Links ]
27. Cypryk K, Kamiñska P, Kosiñski M, Pertyñska-Marczewska M, Lewiñski A. A comparison of the effectiveness, tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Pol J Endocrinol 2007; 58 (4): 314-9. [ Links ]
28. Katz L, Amorim M, Coutinho I, Santos LC. Análise comparativa de testes diagnósticos para diabete gestacional. Rev Bras Ginecol Obstet 2002; 24 (8): 527-33. [ Links ]
29. Institute of Medicine. Weight gain during pregnancy: Reexamining the guidelines. Washington DC: IOM; 2009. [ Links ]
30. Ministério da Saúde. Pré-natal e puerpério. Atenção qualificada e humanizada. Brasília: MS; 2006. [ Links ]
31. Saunders C, Bessa TCCD, Padilha PC. Assistência Nutricional Pré-Natal. In: Accioly E, Saunders C, Lacerda E. Nutrição em Obstetrícia e Pediatria. Rio de Janeiro: Cultura Médica; 2009. p. 103-24. [ Links ]
32. Padilha PC, Accioly E, Veiga GV, Bessa TC, Libera BD, Nogueira JL, et al. The performance of various anthropometric assessment methods for predicting low birth weight in pregnant women. Rev Bras Saude Mater Infant 2009; 9 (2): 197-206. [ Links ]
33. Saunders C, Padilha PC. Diabetes melito na gestação. In: Accioly E, Saunders C, Lacerda E. Nutrição em Obstetrícia e Pediatria. Rio de Janeiro: Cultura Médica; 2009. pp. 193-210. [ Links ]
34. Chagas CB, Ramalho A, Padilha PC, Libera BD, Saunders C. Reduction of vitamin A deficiency and anemia in pregnancy after implementing proposed prenatal nutritional assistance. Nutr Hosp 2011; 26 (4): 843-50. [ Links ]
35. Vítolo MR, Bueno MSF, Gama CM. Impacto de um programa de orientação dietética sobre a velocidade de ganho de peso de gestantes atendidas em unidades de saúde. Rev Bras Ginecol Obstet 2011; 33 (1): 13-9. [ Links ]
36. Padilha PC. Contribuições teórico-práticas para a assistência nutricional pré-natal (tese). Rio de Janeiro. Universidade Federal do Rio de Janeiro; 2011. [ Links ]
37. Santos MMAS, Baião MR, Barros DC, Pinto AA, Pedros PLM, Saunders C. Estado nutricional pré-gestacional, ganho de peso materno, condições da assistência pré-natal e desfechos perinatais adversos entre puérperas adolescentes. Rev Bras Epidemiol 2012; 15 (1): 143-54. [ Links ]
![]() Correspondence:
Correspondence:
Cláudia Saunders.
Instituto de Nutrição Josué de Castro.
Universidade Federal do Rio de Janeiro.
Av. Carlos Chagas Filho, 373.
Centro de Ciências da Saude, Bloco J, 2o andar, sala 26.
21941-590 Ilha do Fundão. Rio de Janeiro-RJ. Brazil.
E-mail: claudiasaunders@nutricao.ufrj.br
Recibido: 2-VI-2013.
1.a Revisión: 10-VIII-2013
Aceptado: 18-IX-2013.