INTRODUCTION
The appropriate estimate of the energy requirements of children with cerebral palsy (CP) is essential in ensuring that their needs are optimally met. This goal is not an easy task, particularly during the stages of maximum physical growth and cognitive development, especially when the child is malnourished and/or has difficulty in eating due to mechanical disorders of swallowing 1,2,3. In many cases, the energy intake in children with CP is insufficient to meet their energy demands compared to healthy children 4. In addition, there are other contributing factors such as difficulty in communicating sensations of hunger and satiety and feeding themselves, and the presence of gastrointestinal disorders such as chronic constipation and gastro-esophageal reflux 5,6. It has been suggested that the resting energy expenditure (REE) and the total energy expenditure (TEE) are significantly lower in children with CP compared to healthy children of the same age, with estimates obtained by indirect calorimetry and doubly labeled water 5,7. Moreover, the equations proposed by the World Health Organization (WHO), based on age, sex and weight, tend to overestimate energy expenditure in children with CP 1,2,7,8.
The main cause of decreased energy expenditure in children with CP is limited physical activity. This limitation in physical activity is influenced by the brain injury itself and by motor dysfunction (impairment of motor skills and muscle tone) 3,4,5,7,9;10,11,12. The main objective was to demonstrate that resting energy expenditure (REE) and total energy expenditure (TEE) are associated with age, anthropometric indicators and body composition in children with cerebral palsy.
MATERIAL AND METHODS
In a cross-sectional analytical study, 79 patients with spastic CP (38 girls and 41 boys) from ages 24 months to 16 years nine months (8 y 5 months ± 4 y 5 months), who attended the outpatient Department of Pediatrics at New Civil Hospital of Guadalajara and were diagnosed and classified by a pediatric neurologist, were included in the study. The study was performed between January and December 2014. The sample was obtained from a previous study on the nutritional status of children with CP and estimated with a confidence level of 95% (α = 0.05) 13. No patients with diagnoses unrelated to CP (Down syndrome, autism, degenerative disorders), receiving medications that may alter the body composition (steroids, thyroxine, anti retrovirals) or with CP of a postnatal origin (traumatic injuries, accidents, tumors, other injuries) were included.
ANTHROPOMETRY
Weight was obtained using a Seca® scale (model 700 Hamburg Germany) with an accuracy of 50 g; each child wore a clean diaper and as little clothing as possible. The child was weighed in the arms of a family member or observer and, subsequently, the adult alone was weighed, and the weight difference results were obtained. The height was obtained using the alternative lower leg length (LLL) measurement with the following equation: (3.26 × LLL) + 30.8. The LLL was measured using a tape measure (Seca®, 206, Hamburg, Germany), by two previously standardized observers, according to the technique of Stevenson 14, and the final value was obtained using the average of the two measurements. The mid upper arm circumference (MUAC) was measured with a flexible tape (Seca®), 206, Hamburg, Germany), at the midpoint of the arm length, from the acromion to the olecranon. The triceps skinfold (TSF) was measured using a Lange® skinfold caliper (Cambridge, Maryland) on the posterolateral inner left arm, in the same spot as the MUAC. Each observer took three measurements and the average was calculated. The sub-scapular skinfold (SSF) was located at the lower angle of the left scapula where a mark was made; skin and adipose tissue were taken a centimeter above and diagonally to the mark; the tips of the caliper were placed, and after each observer took the measurement three times, the average was obtained.
BIOELECTRICAL IMPEDANCE ANALYSIS (BIA)
The bioelectrical impedance was performed using Quadscan 4000 equipment (BodyStat® Ltd., England). After a three-hour fasting period, two electrodes for pediatric assessment were placed on the back of the hand (at the wrist and metacarpal) and two on the dorsum of the foot (metatarsal and ankle). Metal objects that could interfere with the impedance were removed; impedance was set to 50 ohm. The measurement was obtained with the child in dorsal supine position and as relaxed as possible. REE and TEE were estimated according to the equations by BIA QuadScan® 4000 equipment. Previous studies in children with spastic CP have shown good correlation between anthropometric indicators and body composition measurements obtained by BIA using this equipment 2.
STATISTICAL ANALYSIS
An ANOVA and post hoc (Bonferroni) test were performed for the multi-comparisons between age groups (24-71, 72-119 and ≥ 120 months). Pearson correlation coefficients comparing body composition and energy expenditure were also calculated. The data were analyzed using the SPSS version 20 (SPSS Inc., Chicago, IL, USA).
ETHICAL CONSIDERATIONS
The protocol did not put participants at risk and adhered to the Declaration of Helsinki guidelines, in its last correction made during the 64th Annual Assembly organized by the World Medical Association (2013). Adequate information was given to parents about the importance of this study, and the protocol was applied after obtaining signed informed consent. The Bioethics Committee of the New Civil Hospital of Guadalajara approved the project with no. 1344/14.
RESULTS
Table 1 shows the anthropometric and body composition measurements of participants (n = 79); 5.1% of the participants belonged to level I and II of the Gross Motor Function Classification System (GMFCS), 2.5% to level III, 17.7% to level IV and 69.6% to level V. Energy expenditure was expressed in kcal/d, kcal/kg/d and kcal/cm/d. The comparison of REE by sex, female vs male, in kcal/d, was 867 ± 177 vs 911 ± 186, respectively; REE, measured in kcal/kg/d, was 57.3 ± 13.7 vs 53.2 ± 9.4; TEE, in kcal/d, was 1,185 ± 229 vs 1,274 ± 261; and TEE, in kcal/cm/d, was 11 ± 1.3 vs 11.3 ± 1.0. Table 2 shows that significant differences among the age groups were observed in REE in kcal/d and kcal/kg/d and in the TEE in kcal/d. The energy expenditure, expressed in kcal/cm/d, was approximately 11 kcal/cm/d in all age groups. Table 3 highlights a significant direct correlation between REE (kcal/d) and TEE (kcal/d) with fat-free mass calculated using BIA and anthropometry. In addition, there is an inverse and highly significant correlation between REE (kcal/kg/d) and fat-free mass, the results being stronger with the BIA method than with anthropometry. The correlation matrix shows a strong correlation of body composition values obtained by BIA and anthropometric indicators. There was a stronger correlation with the anthropometry method than BIA, with energy expenditure expressed in kcal/d and kcal/kg/d. The obtained R2 showed that fat free mass (FFM), total body water (TBW) and, to a lesser extent, fat mass (FM) explained a high percentage of the REE and TEE direct variability in kcal/d and the inverse in kcal/kg/d.
Table I. Anthropometric characteristics in children with CP (n = 79)
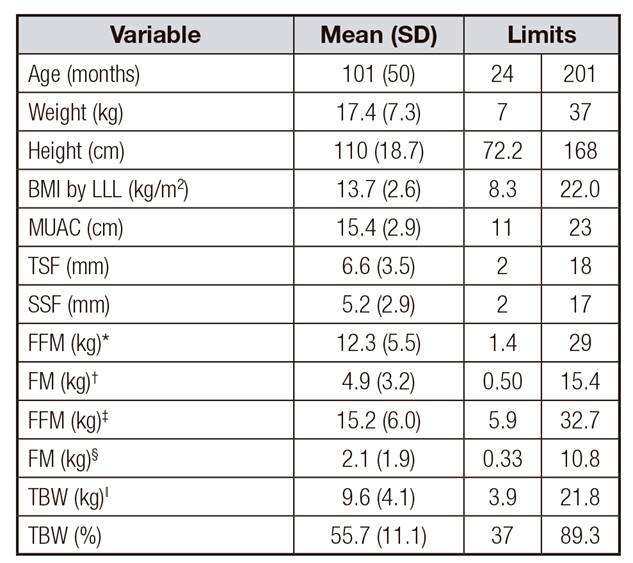
BMI: body mass index; LLL: lower leg length; MUAC: mid-upper-arm circumference; TSF: triceps skinfold; SSF: subscapular skinfold;
*FFM: fat-free mass by bioelectrical impedance analysis (BIA);
†FM: fat mass by BIA;
‡FFM: fat-free mass by anthropometry;
§FM: fat mass by anthropometry;
ǁTBW: total body water by BIA.
Table II. Energy expenditure by age groups

REE: resting energy expenditure; TEE: total energy expenditure.
*ANOVA. Post hoc test (Bonferroni): REE (kcal/d) 24-71 vs 72-119 months, p = 0.001; 24-71 vs ≥ 120 months, p < 0.001; y 72-119 vs ≥ 120 months, p = 0.008. REE (kcal/kg/d) 24-71 vs 72-119 months, p = 0.008; 24-71 vs ≥ 120 months, p < 0.001; y 72-119 vs ≥ 120 months, p = 0.454. TEE (kcal/d) 24-71 vs 72-119 months, p = 0.001; 24-71 vs ≥ 120 months, p < 0.001; y 72-119 vs ≥ 120 months, p < 0.001.
Table III. Correlations among FM, FFM, TBW and energy expenditure in children with spastic cerebral palsy (n = 79)**
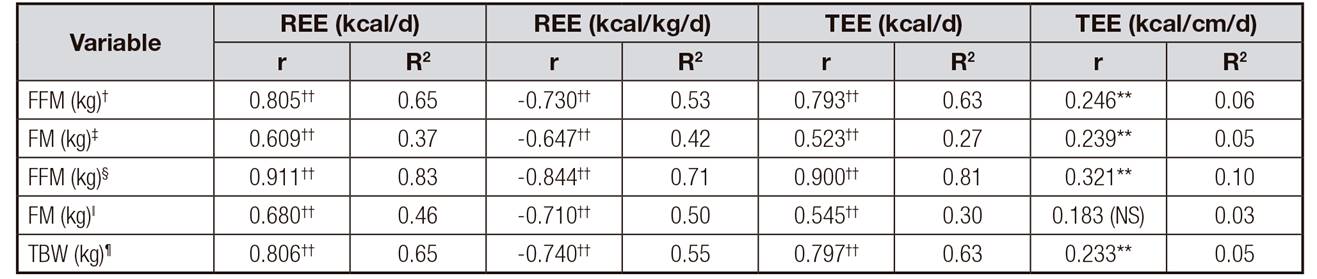
*Pearson correlation;
†FFM: fat-free mass by bioelectrical impedance analysis (BIA);
‡FM: fat mass by BIA; §FFM: fat-free mass by anthropometry;
ǁFM: fat mass by anthropometry;
¶TBW: total body water by BIA; REE: resting energy expenditure; TEE: total energy expenditure;
**p < 0.05;
††p < 0.001; NS: non-significant.
DISCUSSION
To our knowledge, this is the first study completed in Mexico where energy expenditure in 79 children with spastic CP was analyzed. It is noteworthy that Walker et al. 3 showed that in children with spasticity, TEE was 1,367 kcal/d, similar to the level observed in our study (1,231 Kcal/d). Several factors can influence the estimated energy expenditure, regardless of age, for example, outpatient status, constant movements, features of dysfunction (type, distribution and severity) and muscle tone 3,11. Energy expenditure indicators, such as REE expressed in kcal/d and TEE in kcal/d, increased gradually with age (p < 0.001). The REE expressed in kcal/kg/d was higher in the group of 24-71 vs 72-119 months (p = 0.001); also, the REE was higher in the group of 24-71 vs ≥ 120 months (p < 0.001). An inverse relationship was observed: the younger the child, the greater the REE (kcal/kg/d) (p < 0.001). It is likely that, as in healthy children, energy requirements are higher in early stages of life 15. Conversely, it was observed that the TEE, expressed in kcal/cm/d, does not change depending on age and remains stable at 11 kcal/cm/d. These results are consistent with data reported in children with spastic quadriplegia or CP with moderate to severe malnutrition and severe motor dysfunction 2,16,17,18. There has been a reported energy intake ranging from 11 to 14 kcal/cm/d 19. An energy intake of approximately 11 kcal/cm/d would be an appropriate amount for children with CP to maintain an adequate nutritional status.
Other findings of this study suggest that BIA, which has shown high specificity (98.2%) and sensitivity (100%), is a reliable, accurate and relatively easy method for estimating energy expenditure in children with CP 2,7,8,20 compared to indirect calorimetry (IC), which has shown good sensitivity (92.3%) and lower specificity (65.5%) 2. In addition, it was observed that the equations for healthy children, and those proposed by the WHO, based on age, sex and weight, tend to overestimate energy expenditure in children with CP 1,2,7,8,16.
As expected, there was a direct correlation between REE and TEE and the body composition indicators FFM, FM and TBW in children with CP. This finding does not differ from that observed in children without this pathological condition 8,12,21. By contrast, a highly significant negative correlation between REE (kcal/kg/d) and FFM was observed, with a higher correlation with BIA than anthropometry. One possible explanation is that in children with CP, body composition (lean mass) is affected by neurological damage; when the body mass is greater, as in older children, less energy per unit of body weight is demanded. A lack of correlation between body composition and TEE, expressed in kcal/cm/d, was also observed. This finding would suggest that in early life, most likely in children under 36 months of age, the estimated energy expenditure is best expressed in kcal/kg/d, while in older children, as has been shown in other studies, it is better to estimate the TEE in kcal/cm/d for the calculation of diets. Finally, a relevant finding was the highly significant correlations between the indicators of body composition obtained by BIA and anthropometry and energy expenditure indicators. Therefore, both methods (anthropometry and BIA) would be useful predictors for estimating REE and TEE in children with spastic CP.
It has been reported that energy expenditure is lower in children with CP than in healthy children, and a relationship between energy expenditure and the body composition indicators can exist. Walker 3 showed that FFM is a major predictor of energy requirements, and together with motor function, it explains 67% of the variability in energy requirements, which is similar to our result of 65%. Similarly, another study on children with CP with moderate to severe malnutrition showed a high correlation between FFM and TEE (r = 0.748, p = 0.005) 2.
The main strength of this study is that when REE and TEE are expressed in kcal/kg/d and kcal/cm/d, respectively, they allow us to correctly estimate the energy requirements for children with CP. For example, in children less than 36 months of age, energy requirements could be estimated at 62.3 kcal/kg/d, and in older children they could be estimated at 11 kcal/cm/d. The main limitation was the negative values in some measurements of body composition, performed by BIA, particularly in FM (two negative values). If these negative results were a consequence of the severity of the spasticity in these children with CP, then the BIA could not be performed due to a lack of symmetric posture. The negative values were considered as outliers and were eliminated.
In conclusion, it is difficult to determine the estimated energy expenditure in children with CP. As age increases, the indicators of energy expenditure significantly increase. However, estimates of kcal/cm/d do not differ according to sex and age; therefore, the estimation of energy requirements based on height would be a practical and reliable method to maintain an adequate nutritional status. Further research is required; more accurate methods are needed to estimate energy expenditure in children with CP compared with the BIA method and IC method. The use of BIA for estimating energy expenditure is a relatively easy procedure to perform.














