INTRODUCTION
An altered body composition with a high amount of fat mass (FM) and reduced lean mass (LM) has been described in the literature as a risk factor for breast cancer and its recurrence (1). This direct association can be explained by the metabolic mechanisms present in excess body fat, which involve chronic low-intensity inflammation, signaling the metabolic cascades of carcinogenesis (2). This condition, when associated with the presence of carcinogenesis and antineoplastic treatment, seems to alter cell integrity and contribute to a reduction in lean mass (3,4).
The change in the structure and function of the cell membrane can be identified by the phase angle (PA) and also by the reactance (Xc) values (5). PA has been considered an important predictor of clinical prognosis in breast cancer, and low values of this marker are associated with survival and disease recurrence, as they suggest death or decreased cell integrity, reflecting cell function and, consequently, nutritional status (6). Women with breast (7) and ovarian (8) cancer with more advanced clinical staging have lower PA values.
The change in reactance suggests a reduction in the resistive effect produced by the interfaces of tissues and cell membranes, which is related to a dysfunctional membrane status and is correlated with PA (9).
Despite being prognostic parameters described in the literature in several clinical situations (liver diseases [10], critically-ill patients [11]), studies in cancer patients that associate tumor aggressiveness according to the immunohistochemical profile with PA, Xc and leptin in breast cancer survivors are still scarce. Therefore, the present study aims to assess the association between tumor aggressiveness and changes in cell integrity markers (PA and Xc) and leptin in breast cancer survivors over a 5-year follow-up period. Our hypothesis is that women with more aggressive tumors have less favorable changes in cell integrity and leptin levels when compared to those with less aggressive tumors.
MATERIALS AND METHODS
STUDY POPULATION
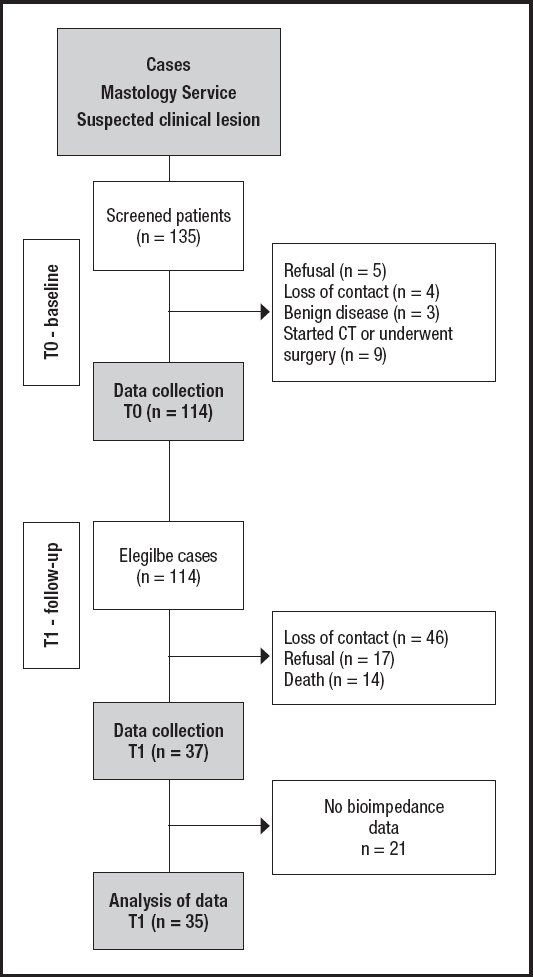
This was an observational, longitudinal, prospective study with 114 women diagnosed with breast cancer, treated at the Mastology Outpatient Clinic of Hospital Geral de Fortaleza (HGF, Brazil). The sample calculation was performed at the previously published baseline (T0) (12), for which variables of interest (clinical stage, body composition and leptin levels) were considered, with a significance level of 5 % and a power of 95 %, assuming a normal distribution of the variables.
Women over 19 years of age, in clinical stage (CS) I to IV, without associated neoplasms and without previous cancer treatment were eligible. The first evaluation took place at the moment of the diagnosis, before any clinical or surgical treatment (T0), and the second moment of collection took place 5 years later (T1).
At follow-up (T1) it was possible to re-establish contact with 68 patients; however, there was a sample loss due to refusal to participate in the reassessment (n = 17) and death (n = 14), thus 37 patients were reassessed in T1 (Fig. 1).
SOCIODEMOGRAPHIC AND CLINICAL PROFILE
For the sociodemographic profile, we collected information on age, marital status, ethnicity, level of schooling, and family income in minimum wages. We collected clinical information from the medical records and through a direct interview, investigating menopausal status, breastfeeding, nulliparity, smoking status, alcohol consumption, family history of cancer, clinical (tumor size, compromised lymph nodes and metastasis) and pathological staging (estrogen (ER) and progesterone (PR) receptors, human epidermal growth factor type-2 receptor (HER2), and percentage of Ki-67).
ANTHROPOMETRIC AND BODY COMPOSITION PARAMETERS
The anthropometric evaluation was performed by measuring the current body weight (BW), height (m), waist circumference (WC) and body composition. BW was measured on a Control digital scale (Plenna®, São Paulo, Brazil), with a capacity of 150 kg and an accuracy of 100 g. Height was measured in an AlturaExata® stadiometer (TBW, São Paulo, Brazil) with a limit of 2.10 m and an accuracy of 1.0 mm. After calculating the body mass index (BMI, kg/m2), the adult participants were classified according to the nutritional status categories recommended by the World Health Organization (13), and the elderly women according to the recommendation established by the North American Dietetic Association (14).
As for WC, an inelastic measuring tape was used to measure it over the umbilicus, using a cutoff point of WC ≥ 80 cm, established by the International Diabetes Federation (15) for Caucasian women of European origin.
Body composition was estimated using tetrapolar electrical bioimpedance (Biodynamics 450, Biodynamics Corporation, USA) with an 800 µA current intensity and 50 KHz frequency, following the evaluation protocol (16) to guarantee the quality of the bioimpedance test information. For this, the patients had fasted for 12 hours, had not consumed alcohol or caffeine, and had not practiced any physical activity in the last 24 hours. The examination was carried out in a place with adequate temperature, 5 minutes after the patient was in the supine position. All women were evaluated with the same equipment at both times (T0 and T1). Lean mass (% LM) and fat mass (% FM), phase angle (PA), resistance (R) and reactance (Xc) were collected.
LEPTIN DETECTION
After a 12-hour fasting period, blood samples (20 mL) were collected in Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA), (1 mg/mL), used as anticoagulant and antioxidant, and these samples were kept on ice and protected from light until the plasma was obtained (1500 g, 15 min, 4 °C), which was stored at -80 °C until the moment of the analyses. The Enzo Life Science® ELISA “sandwich" capture enzyme immunoassays were used for the determination of plasma leptin levels.
STATISTICAL ANALYSIS
Tumor characteristics were dichotomized into: CS I and II, and CS III and IV; tumor size: ≤ 2 cm and > 2 cm; lymph node involvement: present (N+) and absent (N-); tumor aggressiveness: luminal (luminal A, B and hybrid) and non-luminal (HER2+ and triple negative); ER: ER+ and ER-; PR: PR+ and PR-; HER2: HER2+ and HER2-; and % Ki67: ≤ 14 % and > 14 %).
Nominal variables are presented as frequencies and absolute numbers. The continuous variables are expressed in median and interquartile range, regardless of the Gaussian distribution. Normality was tested by the Shapiro-Wilk test, considering non-parametric those with p ≤ 0.05.
To verify the differences in the means of anthropometric variables and body composition between the times T0 and T1, the paired t-test was used for those with normal distribution and Wilcoxon's test for those with a nonparametric distribution. To refine the analysis, we created the delta (∆) variable, which included the differences between the moments T0 and T1. This delta (∆) variable was used too to see changes between T0 and T1 in the means of anthropometric variables and body composition in the different categories of tumor aggressiveness.
The generalized linear model (GLM) of repeated measures, with Bonferroni's adjustment for multiple comparisons and considering the normality of the variables, was used to verify the effect of time and tumor aggressiveness (pT*A) on changes in the anthropometric profile and body composition.
Poisson regression models, with a robust estimator, were structured to assess the prevalence ratio between the tumor aggressiveness variables and changes (∆) in the patients' body composition during follow-up. The dependent variables used were ∆-Xc, ∆-PA and ∆-Leptin, dichotomized by the change during follow-up and having a non-zero increase or decrease as cutoff point. In model 1, tumor aggressiveness (CS, tumor size, N+, ER+, PR+, HER2+, % Ki67 and Luminal type) was the independent variable. In model 2 the variables income, level of schooling and age were added. For these analyses we used the IBM SPSS Statistic software, version 24.0, with a significance level set at p ≤ 0.05 and a 95 % confidence interval (CI).
RESULTS
SOCIODEMOGRAPHIC AND CLINICAL CHARACTERISTICS
During the follow-up of 55.4 months (± 16.62) it was possible to re-establish contact with 59.6 % of the patients, who at T0 were an average of 48.7 years (± 9.42) of age and at T1 of 55.5 years (± 9.7). Most of the participants were of Asian, indigenous or brown ethnicity; they had fewer than nine years of schooling; family income per capita was less than a minimum wage; they had at least one child; they breastfed; never drank alcohol or smoked; lived with a partner and had a family history of cancer (Table I).
ANTHROPOMETRIC PROFILE, BODY COMPOSITION AND LEPTIN AT TIMES T0 AND T1
Regarding the changes in weight status, BMI, WC, body composition and serum leptin of the patients during follow-up (Table II), there was a significant reduction in Xc (p ≤ 0.000) and in PA (p ≤ 0.000) and an increase in the levels of serum leptin (p = 0.01) (Table II).
Table II. Weight status, BMI and WC, body composition, cell integrity and serum leptin levels at T0 and T1, and changes (∆) after follow-up

Results shown as median and interquartile range. aPaired Student's t-test or Wilcoxon's test. Significance level adopted: p < 0.05. T0 = baseline; T1 = second assessment. ∆ = T1-T0. BMI: body mass index; WC: waist circumference; PA: phase angle; R: resistance; Xc: reactance; % LM: percentage of lean mass; % FM: percentage of fat mass.
TUMOR AGGRESSIVENESS AND CHANGES IN BODY COMPOSITION
When analyzing the changes in Xc, PA and leptin levels between T0 and T1 (∆), and of Xc, PA for the time of follow-up associated with aggressiveness (pT*A), we found that patients with N+ (p = 0.02) and % Ki67 > 14 have a reduction in Xc (p = 0.00). Patients with advanced CS (p = 0.02), tumors > 2 cm (p = 0.01), N+ (p = 0.01), non-luminal tumors (p = 0.02), ER- (p = 0.00) and PR- (p = 0.02) show a reduction in PA, and patients with N+ (p = 0.01) show a reduction in leptin during follow-up. The other variables were independently correlated to tumor aggressiveness categorization (Table III).
Table III. Cell integrity status by Xc and PA, and adiposity by serum leptin levels according to markers of tumor aggressiveness at times T0 and T1, and changes (∆) after follow-up

Results presented as median and interquartile range. Δ: T1-T0 in the different categories of tumor aggressiveness; T0: baseline; T1: second assessment. ap T*A: effect of time (T) and tumor aggressiveness on the anthropometric profile and body composition using the Generalized Linear Model of repeated measures and Bonferroni's adjustment for multiple comparisons. Significance level adopted: p < 0.05. PA: phase angle; Xc: reactance; CS: clinical staging — tumor size: < 2 cm and > 2 cm: lymph node involvement: present (N+) and absent (N-); tumor aggressiveness: luminal (luminal A, B and hybrid) and non-luminal (HER2+ and triple negative); ER: ER+ and ER-; PR: PR+ and PR-; HER2: HER2+ and HER2-; and % Ki67: < 14 % and > 14 %.
Table IV shows the results of the regression, demonstrating a lower prevalence of patients who had the PA reduced over follow-up among those in the initial CS (PR: 0.43; CI: 0.20-0.93), with tumors ≤ 2 cm (PR: 0.56; CI: 0.33-0.95), and luminal tumors (PR: 0.068; CI: 0.01-0.95), both before and after adjusting for income, level of schooling and age. However, there is a higher prevalence of women with reduced PA over the 5-year follow-up among patients with ER+. Initial CS is associated with a higher prevalence of increased leptin (PR: 6.81; CI: 0.00-0.00) before and after adjustment for income, level of schooling and age.
Table IV. Prevalence ratio of changes in Xc, PA and Leptin levels during follow-up (T1-T0)

Poisson regression with robust estimator. Results shown as prevalence ratio (PR) and 95 % confidence interval (CI). Model 1: Δ = T1-T0: dependent variable; CS, tumor size, N+, ER+, PR+, HER2+, % Ki67 and Luminal: independent variables. Model 2: Δ = T1-T0: dependent variable; CS, tumor size, N+, ER+, PR+, HER2+, % Ki67 and Luminal: independent variables adjusted for age, income and level of schooling. Significance level set at p < 0.05. CI: some values are zero because of small numbers of patients at the second assessment (T1). PA: phase angle; Xc: reactance; CS: clinical staging — tumor size ≤ 2 cm and > 2 cm; lymph node involvement: present (N+) and absent (N-); tumor aggressiveness: luminal (luminal A. B and hybrid) and non-luminal (HER2+ and triple negative); ER: ER+ and ER-; PR: PR+ and PR-; HER2: HER2+ and HER2-; and % Ki67 ≤ 14 % and > 14.
DISCUSSION
The present study investigates the association between tumor aggressiveness, body composition and cell integrity in women with breast cancer during an average follow-up of 4.6 years, and demonstrates that the patients did not change weight, BMI, WC and body composition, but had changes in Xc, PA and leptin levels influenced by tumor aggressiveness. These markers are widely known as direct measures of cell integrity and adiposity and are involved in the clinical prognosis of cancer, survival, and mortality (6,17,18).
Women diagnosed with breast cancer become more careful with their lifestyle (19) and 30 to 60 % of them increase their consumption of fruits and vegetables, and reduce their intake of red meat, fats, and sugary foods (20), changes that allow the maintenance of body composition and can explain the preservation of anthropometric measures and body composition of the patients in our sample.
Despite remaining with the same body composition, patients have lower Xc and AF, and higher leptin values during follow-up. Xc and PA are pure measures of bioimpedance assessment, which means they are more adequate to speak about cell integrity and body composition (21). PA is understood as a marker of cell membrane integrity, is associated with nutritional status (7), and low values suggest cell death or reduced integrity (22).
In patients with cancer, PA has been described as a survival predictor regardless of the clinical factors established for the prognosis (6), and stands out for its association with higher mortality and risk of recurrence (17). The indicated mean PA score for patients with breast cancer is 5.6° (1.5 to 8.9) (6). Those with PA ≤ 5.6° have a shorter survival when compared to patients with a PA > 5.6°. In our study, the mean PA of patients at T0 was 6.3°, with a 0.7° decrease during the follow-up. The reduction in PA was present in patients with tumors with more advanced CS, tumor size > 2 cm, N+, ER-, PR- and non-luminal, and therefore more aggressive ones. This finding corroborates the literature, which shows that tumors with more aggressive characteristics promote changes in cell structure in a systemic way, favoring a worse clinical prognosis, reduced survival, and increased disease recurrence rates for these women (18). In agreement with our findings, Tyagi et al. (8), studying PA in women with breast cancer, found that the lower the PA values, the bigger the tumors, with N+ and presence of metastases. According to the authors, this is because neoplastic cells damage cell junctions, leading to loss of homeostasis between ions, changes in transport in the plasma membrane, increased production of aerobic lactate, and insertion of new proteins in the cell membrane, leading to worse integrity.
We also found that women with the initial CS (PR: 0.43, CI: 0.20-0.93; p = 0.03), tumors < 2 cm (PR: 0.56, CI: 0.33-0,95; p = 0.03), and luminal tumors (PR: 0.068, CI: 0.01-0.95; p = 0.04) have a lower prevalence of PA reduction during follow-up, suggesting that more favorable clinical or histopathological characteristics are associated with better cell integrity. Studies that assess the effect of tumor aggressiveness on PA have described the effect for the head and neck cancer site (18), but are still incipient in breast cancer, which allows us to affirm that our findings greatly contribute to the scientific knowledge of this association.
In addition to phase angle, Xc has also been studied as a marker of body composition and cell integrity, with a low Xc being described as indicative of a reduction in the resistive effect produced by the interface of tissues and cell membranes, in association with a dysfunctional membrane status (23). In our findings, Xc had a behavior similar to that of phase angle, decreasing after follow-up, and this change was influenced by the time associated with aggressiveness, with the markers Ki67 > 14 % and N+ being associated with a reduction in reactance.
The reduction in Xc in cancer patients is related to the release of inflammatory cytokines derived from the tumor microenvironment (24) and altered homeostasis (25). This chronic and continuous inflammatory environment, present in our patients since the diagnosis due to the neoplasm and also to excess weight and fat mass, generates high levels of reactive oxygen species and proinflammatory cytokines (26), which stimulate mutagenic agents, react with DNA, alter cell integrity, and favor mutations in epithelial cells and the stroma (4). Considering our findings, we understand that since the inflammatory process has been present since tumor genesis, Xc is configured as a sensitive marker, which can be used for purposes of monitoring and early detection of changes in cell membrane integrity in breast cancer.
In addition to body composition and cell integrity, leptin has been suggested as a potential prognostic biomarker in breast cancer (27). This adipokine has its synthesis and plasma levels proportional to fat mass (28) and has several biological activities, including breast tumorigenesis (29). Leptin is produced mainly by local adipocytes, but also by epithelial tumor cells and other cells within the tumor stroma; it plays an essential role in the pathogenesis and metastatic potential of breast cancer (30), regulating the stages of carcinogenesis and the inflammatory process of cancer, and attenuating the apoptotic response. Additionally, this adipokine is involved in the regulation of the estrogen receptor (ER-α), interfering with the growth and progression of hormone-dependent breast cancer, stimulating the expression of aromatase and estrogen synthesis, decreasing the effectiveness of hormonal therapies (31). As a result, leptin has been identified as sensitive and predictive of breast cancer survival and mortality regardless of staging (32), and our findings corroborate this statement, since we found a higher prevalence of increased leptin in the initial CS — % Ki67 ≤ 14 and luminal tumors.
Goodwin et al. (33), in a prospective study, found that serum leptin at diagnosis was not associated with disease-free survival (DFS) or overall survival (OS) at 6 years of follow-up, but with 12.1 years of follow-up, higher leptin levels correlated with worse DFS and OS.
In the last decades, the survival rates of patients with breast cancer have steadily increased (34), which places the prevention of late recurrence, at 5 to 20 years after diagnosis, as a challenge among health professionals (35). Thus, the identification of factors associated with late recurrence, such as leptin levels, in breast cancer survivors can help plan individualized treatment strategies and preventive measures related to lifestyle, such as fat mass management.
Our findings improve the discussion of the state of the art that involves lifestyle in preventing breast cancer recurrence, indicating the need for attention to cell composition and integrity since the diagnosis, aiming to minimize negative outcomes for these women.
We emphasize as a limitation of our study the loss of patients to follow-up, which can be explained by the low level of schooling of the participants, who do not understand the importance of studies such as this and their impact on the health care of women who survive breast cancer. Moreover, during follow-up, between the years 2011 and 2016, there was a migration from landline phones to mobile phones in Brazil, which led to significant loss of contact. However, it is essential to emphasize that this is an unprecedented follow-up study with Brazilian women, aiming to assess body composition and cell integrity along with tumor aggressiveness.