INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a disease in which there is excessive fat deposition in hepatocytes due to hepatoprotective factors other than a history of significant alcohol consumption, drug use, hepatitis C virus infection, or other diseases such as starvation (1). Fatty liver can cause liver damage that leads to inflammation (non-alcoholic steatohepatitis [or NASH]) or liver scarring (cirrhosis) (2). NAFLD is a common worldwide disease with a 10-30 % prevalence in different countries. NAFLD is also increasingly recognized as the cause of elevated levels of liver enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), or γ-glutamyl transferase (GGT) (3). Several studies have shown that NAFLD is a significant risk factor for peripheral vascular disease, diabetes mellitus, cardiovascular disease, and renal disease, which have serious consequences (4-6).
Insulin resistance (IR) (7,8) is the inadequate response of adipocytes, muscle cells, and hepatocytes to normal insulin concentrations when the pancreas has no pathological abnormality. The liver is one of the main sites for the control of metabolic homeostasis. Metabolic diseases such as IR, type 2 diabetes (T2D), dyslipidemia, and NAFLD are linked through molecular biochemistry and complex immune mechanisms (9,10). Hyperinsulinemia is caused by β-cell efforts to overcome IR by enhancing insulin release. High caloric intake impairs insulin receptor signaling, resulting in defective inhibition of FFA release from adipocytes and defective nitric oxide (NO) release (11). Thus, IR and inflammation form a vicious circle in the presence of lipotoxicity, each contributing to the other and accelerating the development of NAFLD and other metabolic disorders (12). In obese and lean subjects, high IR was found to be the most critical predictor of NAFLD (13). Studies have shown that serum insulin levels are strongly associated with ballooning and hepatic lobular inflammation (14).
The aforementioned studies suggest that IR increases the risk of NAFLD and that IR may cause steatosis (15-17). In addition, the triglyceride glucose (TyG) index, an early marker of IR, is considered a cheap and reliable proxy for IR (18). An elevated TyG index has been reported to reflect more severe IR and is associated with all-cause and cardiovascular disease-induced mortality (19). In recent years, the relationship between TyG and NAFLD has been a hot research topic. However, the relationship between the TyG index and NAFLD risk remains limited. Most studied populations are adolescents and middle-aged adults (20-22). To the best of our knowledge, the relationship between the TyG index, NAFLD, and mortality in older Chinese adults has not been reported.
Therefore, this study aimed to explore the association between triglyceride glucose index and the occurrence and mortality of NAFLD in elderly inpatients.
MATERIALS AND METHODS
STUDY DESIGN AND POPULATION
This prospective observational study included elderly inpatients admitted to the Department of Endocrinology at the Linyi Geriatrics Hospital Affiliated to Shandong Medical College between August 2020 and April 2021.
Inclusion criteria were: 1) diagnosis of NAFLD according to the guidelines proposed by the APWG guidelines (23); 2) age above 65 years; and 3) availability of liver ultrasound. Exclusion criteria were: 1) having hepatic viral infection, autoimmune hepatic disease, other chronic hepatic diseases, carcinomatous cachexia (concerning the clinical history), severe infections, acute myocardial infarction, and acute cerebral infarction; and 2) long-term use of steatogenic medications and exceptionally high alcohol intake (more than 140 g per week in men and more than 70 g per week in women).
The study was approved by the Ethics Committee of the Affiliated Hospital of Shandong Medical College (Linyi Geriatric Hospital) [ SDYZFSYY2020002] and was conducted by the Declaration of Helsinki. All patients signed an informed consent form to verify consent and compliance.
PROCEDURES
Using an ultrasound machine, trained sonographers performed liver ultrasound (Toshiba, Tokyo, Japan), and NAFLD was diagnosed by ultrasound imaging. The study objectives and experimental data were single-blinded to the sonographer. Images were acquired in a standard fashion, with the patient in the supine position with the right arm raised above the head.
Detailed medical history and lifestyle information, including age, sex, smoking, and drinking status, was confirmed by a trained physician from medical records. Current smoking status was defined as ‘yes' if the patients had smoked at least one cigarette per day or seven cigarettes per week in the past six months. Current drinking status was defined as ‘yes' if the subject had consumed alcohol at least once a week in the past six months. Blood pressure (BP) in the nondominant arm was measured in the seated position after 5 minutes of rest using an automated electronic instrument (OMRON HEM-752 FUZZY' Omron Corporation, Dalian, China). Three measurements were taken one minute apart, and the average of the three measurements was used for analysis. Measured BP above 140/90 mmHg or being treated with antihypertensive therapy was considered a criterion for hypertension. An experienced physician measured body height, weight, and waist circumference (WC) with an accuracy of 0.1 cm, 0.1 kg, and 0.1 cm, respectively, and participants wore light indoor clothing while standing without shoes. Body mass index (BMI) (kg/m2) was calculated as weight (kg) divided by height (m2). All patients fasted overnight before blood samples collection. Fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) measurements were analyzed and determined by an automated analyzer according to standard methods. TyG index was calculated according to the established formula: TyG = Ln [TG (mg/dl) × FPG (mg/dl) / 2].
Participants were followed up for six months, and survival was collected. Follow-up was conducted by telephone, and there were no missed visits.
STATISTICAL ANALYSIS
The SAS version 9.1 (SAS Institute, Cary, NC) was used for database management and statistical analysis. Data for continuous variables were expressed as mean ± standard deviation (SD), and data for categorical variables were expressed as numbers (percentages). The Kolmogorov-Smirnov test was used to test the normal distribution. Comparisons of means and proportions were performed using Student's t-test and the Chi-square test, respectively. In addition, logistic regression analysis was used to evaluate the association between the TyG index and NAFLD. Receiver operating characteristic (ROC) curves were used to determine the optimal TyG index cut off point for predicting NAFLD. Cox regression models with hazard ratios (HRs) and 95 % CIs were conducted to examine the association between TyG index and mortality. A two-sided p < 0.05 was considered statistically significant.
RESULTS
A total of 264 patients were enrolled (mean age, 86.6 ± 6.4 years; male/female, 203/61), with 52 (19.7 %) of them having NAFLD. There were significant differences in TG, ALT, TyG index, BMI, and WC between NAFLD and non-NAFLD patients (all p < 0.05). However, age, sex, smoking status, alcohol consumption status, SBP, DBP, HbA1c, FPG, TC, LDL-c, HDL-c, AST, T2D, and hypertension between NAFLD and non-NAFLD patients were comparable (all p > 0.05). Moreover, mortality in NAFLD patients (20.8 %) was significantly higher than in non-NAFLD (8.8 %) patients (p = 0.006) (Table I). With the increase of the TyG index tertile, the occurrence of NAFLD increased significantly (ptrend < 0.001) (Fig. 1).
Table I. Characteristics of NAFLD and Non-NAFLD in older adults.

SBP: systolic blood pressure; DBP: diastolic blood pressure; HbA1c: hemoglobin A1c; FPG: fasting plasma glucose; TC: total cholesterol; TG: triglyceride; LDL-c: low-density lipoprotein cholesterol; HDL-c: high-density lipoprotein cholesterol; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TyG: triglyceride and glucose index; BMI: body mass index; WC: waist circumference; T2D: type 2 diabetes.
A multivariate logistic regression analysis showed that TyG (OR = 3.889; 95 % CI: 1.134-11.420; p = 0.014) and ALT (OR = 1.064; 95 % CI: 1.012-1.118; p = 0.015) were independent risk factors for NAFLD (Fig. 2 and Table II). ROC curve analysis of the TyG index for NAFLD showed an AUC of 0.727 [(p < 0.001); 95 % CI: 0.645 to 0.809], with sensitivity = 80.4 %, and specificity = 57.8 % at cut off = 8.71 (Fig. 3).

Figure 2. Independent factors for NAFLD by multivariable logistic regression analysis. BMI: body mass index; WC: waist circumference; ALT: alanine aminotransferase; TyG: triglyceride and glucose index.
Table II. Risk factors associated with NAFLD.
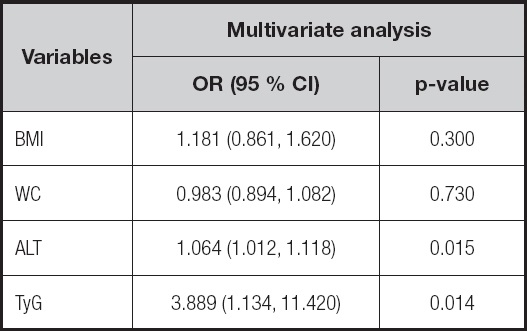
BMI: body mass index; WC: waist circumference; ALT: alanine aminotransferase; TyG: triglyceride and glucose index.
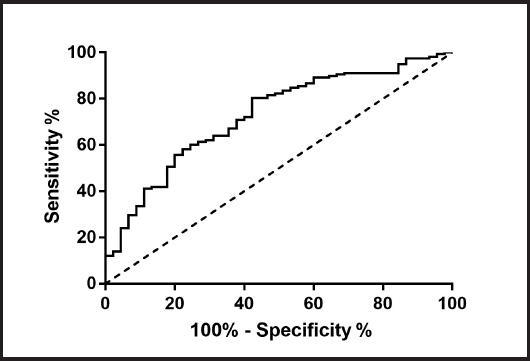
Figure 3. ROC curve analysis of TyG for NAFLD. AUC = 0.727 (p < 0.001); 95 % CI: 0.645-0.809; TyG cut off point: 8.71; Youden index: 0.342; sensitivity: 80.4 %; specificity: 57.8 %.
Furthermore, the Kaplan-Meier survival curve for TyG > 8.71 was significantly higher than that of the TyG < 8.71 group (p < 0.001) (Fig. 4). A Cox proportional hazard regression model showed that, after adjusting for age, sex, smoking, drinking, hypertension, and T2D, TyG > 8.71 (HR = 3.191; 95 % CI: 1.347 to 7.560; p < 0.001) was an independent risk factor for mortality (Fig. 5).

Figure 4. Survival curves of the study population according to the TyG cut off point. Log-rank test (p < 0.001).
DISCUSSION
This study suggested that the TyG index may be associated with the occurrence and mortality of NAFLD in elderly inpatients. Therefore, the TyG index may be a potential and non-invasive indicator to identify NAFLD.
Several studies have confirmed that IR is closely associated with the development and progression of NAFLD (24,25). Elevated IR can lead to changes from normal liver to NAFLD to non-alcoholic steatohepatitis (NASH) (20). The TyG index is associated with IR in muscle (26), and an increase in IR in muscle leads to glucose flow to the liver and a relatively low IR in the liver leading to fatty liver (27). Therefore, the TyG index is an inexpensive and reliable IR surrogate that plays a vital role in the development and progression of NAFLD (28). To our knowledge, our study is the first to focus on the effect of the TyG index on morbidity and mortality in NAFLD in Chinese elderly people, and elevated TyG remained an independent risk factor for death in the Chinese elderly patient population after adjusting for potential confounders. A retrospective cohort study showed that after adjusting for possible confounders (including sex, BMI, SBP, DBP, HbA1c, ALT, AST, and HDL-c), the prevalence of NAFLD in older adults aged 60 years or more senior was 12.1 % (29), which was lower than in our study (19.7 %). A higher TyG index has also been reported to be associated with a higher risk of NAFLD, which is consistent with our results (29). In this study, we identified an optimal TyG index threshold of 8.71 for predicting NAFLD morbidity in older Chinese adults, which is similar to other research (29), taking race, sex, age, and sample size into account.
This study also has some limitations. First, although ultrasonography showed high sensitivity and specificity in the diagnosis of moderate and severe fatty liver (29), it is still not accurate enough compared to liver biopsy-based diagnosis and may lead to the underdiagnosis of mild fatty liver. Therefore, we may only reveal a significant association between TyG index and moderate and severe fatty liver. Second, the sample size of this study was small, and the data were not analyzed according to gender. Finally, all participants in the study were Chinese, so the results may not apply to other ethnic groups.
In conclusion, the TyG index may be associated with the occurrence of NAFLD and mortality in elderly inpatients. TyG index may be a valuable indicator for NAFLD prediction and help prevent the development of NAFLD at an early stage.
















