Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
The European Journal of Psychiatry
versión impresa ISSN 0213-6163
Eur. J. Psychiat. vol.30 no.2 Zaragoza abr./jun. 2016
Association study of the TPH2 Gene with Major Depressive Disorder in the Han Chinese Population
Jing Dua; Zhaofeng Zhanga; Weidong Lib; Lin Heb; Jianhua Xu and Yongyong Shib
a Key Lab. of Reproduction Regulation of NPFPC, SIPPR, IRD, Fudan University, Shanghai. China
b Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Shanghai Jiao Tong University, Shanghai. China
This work was supported in part by National Basic Research Program of China and Shanghai Committee of Science and Techno-logy, China (No. 2010CB529600) and Shanghai Committee of Science and Technology, China (No. 11ZR1431100).
ABSTRACT
Background and Objectives: Tryptophan hydroxylase 2 (TPH2) catalyzes the rate-limiting step in serotonin biosynthesis in the nervous system. Several variants of human TPH2 have been reported to be associated with a spectrum of neuropsychiatric disorders such as unipolar major depression, bipolar disorder and suicidality etc. Recent studies suggested that two variants (T212 and A375) in the exon 7 and exon 9 were associated with major depressive disorder (MDD).
Methods: To replicate these findings, two polymorphisms located in exons 7 and 9 of TPH2 (rs7305115 and rs4290270, respectively) were analysed by DNA sequence in the case-control sample study in 191 MDD and 191 healthy volunteers. Statistical analyses were carried out using the program SPSS. The comparison of allele and genotype frequencies of each polymorphism between case and control groups was carried out on the online software SHEsis. All subjects were unrelated southern Han Chinese.
Results: No difference was observed on the allelic or genotypic distribution of TPH2 gene polymorphisms between the groups. However, the two-marker haplotype covering components T212 (rs7305115) A and A375 (rs4290270) T were observed to have a significantly protective effect MDD in female (corrected p = 0.0032; OR = 0.241[95% CI = 0.099-0.587]).
Conclusions: The results suggest that TPH2 might be associated with a lower risk of female MDD. However, confirmatory studies in independent samples are needed.
Keywords: TPH2; Polymorphisms; Major depressive disorder.
List of abbreviations
MDD: major depressive disorder.
5-HT: Serotonin (5-hydroxytryptamine)
ADHD: attention-deficit/hyperactivity disorder
TCAs: tricyclic antidepressants
SSRIs: selective serotonin reuptake inhibitors
MAOIs: monoamine oxidase inhibitors
TPH: Tryptophan hydroxylase
TPH1: Tryptophan hydroxylase 1
TPH2: Tryptophan hydroxylase 2
5-HTT: serotonin transporter
5-HTR: serotonin receptor
MAOA: monoamine oxidase A
SNPs: single nucleotide polymorphisms
ESE: exonic splicing enhancer
OR: odds ratio
SR proteins: Ser/Arg-rich proteins
SD: standard deviation
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a monoaminergic central neurotransmitter that is widely distributed in the human central nervous system and in certain peripheral tissues. 5-HT has been shown to influence a variety of peripheral and brain physiological functions, including sleep-wake cycle, mood, appetite, aggression, neuroendocrine regulation, neurogenesis and haemostasis1-3. Dysregulation of brain serotonergic homeostasis has been implicated in many neuropsychiatric disorders, including major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), autism, aggression and suicidal behaviour, bipolar disorder and anxiety disorder1. Most antidepressant drugs, including many tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and monoamine oxidase inhibitors (MAOIs), have their effect by increasing levels of extracellular 5-HT by inhibiting its reuptake or metabolism4-6. Serotonin homeostasis is modulated mainly by its receptors, transporter, and enzymes of its biosynthetic pathway. Genes encoding proteins involved in the serotonergic system, including tryptophan hydroxylase (TPH), serotonin transporter (5-HTT), serotonin receptor (5-HTR) and monoamine oxidase A (MAOA), are major genes in association studies of affective disorders7-9.
TPH is the rate-limiting enzyme in the serotonin biosynthetic pathway and plays an important role in the regulation of serotonin function10. Up to now, two different TPH enzymes are expressed by two distinct genes: TPH1 and TPH2 genes. TPH1 has been thought as the sole rate-limiting enzyme for brain serotonin synthesis11 studied for several decades. Recently researchers found that TPH1 expresses predominantly in the periphery12 while tryptophan hydroxylase-2 (TPH2) is exclusively expressed in neuronal cell types and is the predominant isoform of serotonin in the brain 13. TPH2 then has been identified as a neuronal-specific isoform which controls brain serotonin synthesis14,15. Currently, more than 430 single nucleotide polymorphisms (SNPs) in TPH2 have been identified in cohorts of various neuropsychiatric disorders or in general population. A number of them are coding mutations with unknown functions15-18.
MDD is complex, polygenic disorders with genetic, environmental and biochemical influences as potential contributing factors, and affect tens of millions of people with enormous social and economic impact. Given the widespread and disabling nature of the illness, MDD represents a rising public health concern. It is estimated that MDD will become the second leading cause of disability globally by the year 202019. The overall contribution of genetic factors in the origin of these diseases is approximately 40%. The genetic pathogenesis of MDD remains unclear. The identification of the role of TPH2 in brain serotonin synthesis has opened a new area to explore the molecular and genetic mechanisms of serotonin-related neuropsychiatric disorders. Therefore, functional characterization of TPH2 may ultimately provide important insights into the pathophysiology of these disorders. Two polymorphisms located in exons 7 and 9 of TPH2 (rs7305115 and rs4290270, respectively) were shown to be associated with MDD20. However, there have been no consistent findings concerning the relationship between two polymorphisms and MDD as well as therapeutic response in Asian population21. Following these inconsistent results, in this study we investigate the association between these two genetic variants of the TPH2 gene and MDD in a Chinese Han population.
Materials and methods
Samples
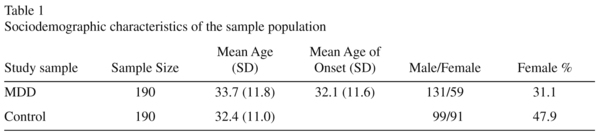
The sample set consisted of 190 unrelated MDD cases (131 males and 59 females), and 190 normal controls (99 males and 91 females) recruited from the Chinese Han population. The mean age of MDD cases was 33.7 years (± 11.8) and the mean age of the controls was 32.4 years (± 11.0) (Table 1). All subjects were born in Shanghai. All patients were interviewed by two independent psychiatrists and were diagnosed strictly according to DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). All subjects gave an informed consent, the details of which were reviewed and approved by the local ethical committee. Controls were randomly selected from the Shanghai general population. The study complied with the guidelines of our local Medical Ethical Committee and all participants recruited in this study provided written informed consents.
Genotyping
Genomic DNA was extracted from venous blood with the QIAamp DNA Blood Kit (QIAGEN, Hilden, Germany) according to the manufactures' instructions. Genotyping was performed without knowledge of the clinical status of the subjects. The sequences of the PCR primers and cycling conditions are given in Table 2. PCR was carried out in a 15 ul reaction mixture containing 10 ng of DNA, 10 pmol of each primer, 2.5 mM MgCl2, 0.2 mM dNTP and 0.25 U Taq DNA polymerase (Sigma, St. Louis, MO, USA). All reactions had an initial denaturation step of 3 min at 94oC, followed by 35 cycles of 94oC for 30s denaturation, 55oC for 30s annealing and 72oC for 1 min, and finally at 72oC for 10 min on a Gene Amp PCR system 9700 (Applied Biosystems, Foster City, CA). Preparation of DNA for sequencing included incubation of PCR products with 0.1 U of shrimp alkaline phosphatase (Roche, Basel, Switzerland) and 0.5 U of exonuclease I (New England Biolabs Inc., Beverly, MA) at 37oC for 45 min, followed by heat inactivation at 85oC for 20 min. The PCR products were sequenced using an ABI Prism BigDye Terminator Cycle Sequencing Kit, version 3.1 (Applied Biosystems, Foster city, CA). The sequences were analysed in an ABI PRISM model 3100 DNA Sequencer (PE Applied Biosystems, Perkin-Elmer) to determine the genotypes of variation at the same position on both forward and reverse sequences. Any differences were resolved by re-genotyping samples (with an overall error rate < 0.05%).
Prediction of ESE site disruption:
Change in ESE motifs due to single base substitutions were calculated by use of the ESEfinder3.0 program (http://rulai.cshl.edu/ cgi-bin/tools/ESE3/esefinder.cgi?process=home)13.
Statistics
Statistical analyses were carried out using the program SPSS (version 19.0). The odds ratio (OR) and their 95% confidence intervals were estimated for the effects of alleles. Haplotype distribution was estimated using the program UNPHASE22. The comparison of allele and genotype frequencies of each polymorphism between case and control groups was carried out on the online software SHEsis (http://202.120.7.14/analysis/myAnalysis.php)23. In all of the analyses, p < 0.05 was considered statistically significant, after Bonferroni correction. Power analysis of our sample was performed using the G*Power 3 programme24.
Results
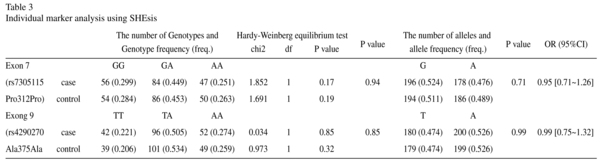
To examine the association between MDD and the polymorphisms in the TPH2 gene, we detected genotype and allele frequencies of two SNPs, T212 (rs7305115) A and A375 (rs4290270) T in the 191 MDD patients and the 191 healthy controls. The distribution of the two polymorphisms was in Hardy-Weinberg equilibrium (Table 3). The data for genotypes and allele frequencies are shown in Table 3. As shown in Table 2, no significant differences in allele or genotype frequencies of the two polymorphisms between the case groups and the control group were observed.
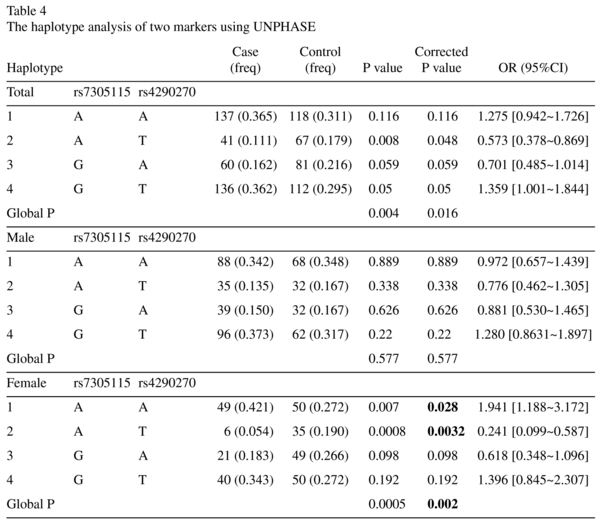
Haplotypes with probabilities greater than 1% accounted for the majority of haplotype diversity. The A-T Haplotype which was more frequent in female controls, was observed to be significantly associated with protect MDD in female (OR = 0.241, p = 0.0008, corrected p = 0.0048) (Table 4). We adjusted the p value using the Bonferroni correction so as to control type 1 error.
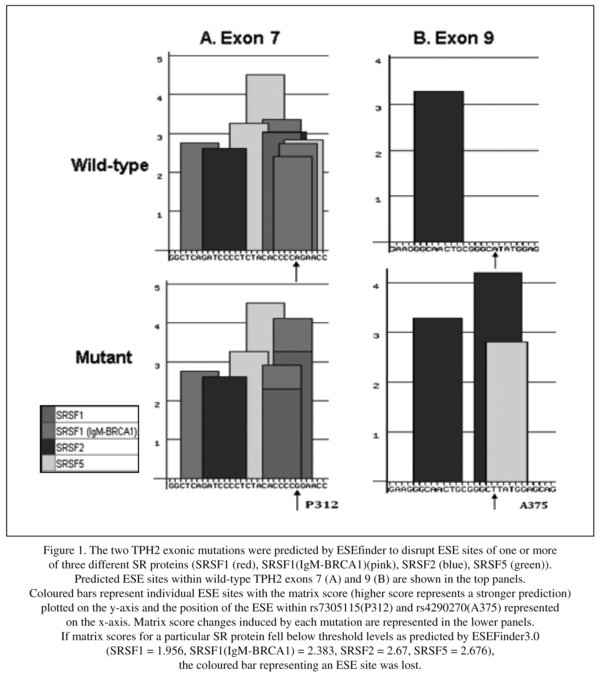
ESEfinder program was used to identify potential binding sites splicing factors in exon 7 of TPH2 gene. We found some changes of putative ESE motifs, SF2/ASF, SC35 and SRp40, in TPH2 predicted by ESEfinder (Figure 1).
In this study, power analysis showed that the statistical power of our sample to detect a significant association (p < 0.05) was 99.8% in genotypic comparisons for MDD when a large effect size (w = 0.8) was presumed. This indicates that the sample size in our study was sufficient to achieve a relatively low risk of a type II error.
Discussion
Psychiatric disorders such as MDD place a large burden on society and health service. Every year, almost one million people die from suicide (http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/). Numerous genetic studies in search for the genes involved in mental disorders have been performed.
Components of the serotonin system are being studied as risk factors in MDD, obsessive-compulsive disorder and autism1 and also play an important role in the clinical effectiveness of antipsychotic drugs25. Alterations in the5-HT system have also been related to specific symptoms and treatment of MDD. Recently, the associations between TPH2 variants, methylation and MDD had been identified in multiple populations26-28. However, the contribution of the TPH2 gene to MDD is controversial and the relationship between TPH2 gene and MDD remains elusive.
In the present study, the frequencies of all the haplotypes were larger than 3%. Initially, we conducted a total association study without consideration of gender. To explore whether a gender difference existed in the association between MDD and TPH2, we conducted a comparison within different gender groups. The haplotypes at the rs7305115 and the rs4290270 was significantly associated with female MDD, where a gene-sex interaction was observed. The haplotypes A/T, which were more frequent in female control subjects (19.0%) than in patients (5.4%), might be significant protective effect against MDD in female (corrected p = 0.0032).Conversely, the haplotype A/A was more frequent in patients (42.1%) than in control subjects (27.2%), suggesting that haplotype G/G is a risk haplotype for female MDD (corrected p = 0.028). All these results suggest that at least one susceptibility locus for MDD lies within, or very close to, the region spanning TPH2 genes in Chinese subjects.
Although our evidence could be a chance finding, it fits well with the evidence of gender differences in the risk of MDD which shows that females are more often affected than males. Women are twice as likely to suffer from depression as men. Women with MDD experience an earlier age of onset, a greater variety of symptoms and an increased number of episodes of depression compared to men29. Many studies showed that female hormones (e.g. estrogen) treatment have antidepressant effects30,31. Ovarian hormones can interact with serotonergic function to influence affect. Estrogen has been shown to increase the density of 5HT2A receptors in brain regions associated with mood32 and can facilitate serotonergic transmission by enhancing serotonin synthesis and/or decreasing serotonin reuptake thereby alleviating depressive symptoms33.
None of the individual SNPs showed positive association whereas haplotype analysis gave a significant association with MDD. This may be explained by the fact that haplotype analysis has a higher power than individual genetic markers in association analysis, since haplotype analysis takes into account the correlation between the individual markers34.
The two SNPs investigated are synonymous. rs7305115 has been found to influence gene expression in post-mortem human pons in which the A allele was associated with higher levels of expression35. rs4290270 may also have functional effects on gene expression32-33. This may be due to exon skipping or alterations in mRNA stability. There is increasing evidence that many human disease genes harbour exonic mutations that affect pre-mRNA splicing36-39. Bioinformatics analysis revealed that the rs7305115 variant changes the overlapping ESE motifs for SRSF2 (SC35) and SRSF5 (SRp40) to motifs for SRSF1 (SF2/ASF) and SRSF1 (IgM-BRCA1), while overlapping SC35 and SRp40 motifs are formed by the rs4290270 variant.
Our results raise the possibility that TPH2 gene variants might be involved in the development of MDD. However, several issues should be noted in the present study. The major limitations of the present study were the relatively small sample size which is liable to result in a stratification bias. Protective haplotype could vary according to ethnic differences. Thus, further studies using a larger number of subjects in different ethnic groups should be performed to determine whether the TPH2 gene haplotype may be truly involved in the development of MDD. Also, the choice of SNPs was based on previous research and focused only on the exons of the TPH2 gene. Accordingly it is necessary that further research provide more complete coverage of the TPH2 gene and investigate other variants that may have an effect on the expression of the gene.
Further work with large sample size or from different populations and corresponding functional analysis is still required to fully elucidate the exact role of TPH2 in the pathogenesis of MDD and other psychiatric disorders.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
1. Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998; 44(3): 151-62. [ Links ]
2. Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003; 4(12): 1002-12. [ Links ]
3. Hynes M, Rosenthal A. Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol. 1999; 9(1): 26-36. [ Links ]
4. Veenstra-VanderWeele J, Anderson GM, Cook EH, Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol 2000; 410(2-3): 165-81. [ Links ]
5. Malhotra AK, Murphy GM, Jr., Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004; 161(5): 780-96. [ Links ]
6. Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999; 283(5400): 397-401. [ Links ]
7. Mössner R, Döring N, Scherag A, Schäfer H, Herpertz-Dahlmann B, Remschmidt H, et al. Transmission disequilibrium analysis of the functional 5-HT3A receptor variant C178T in early-onset obsessive compulsive-disorder. J Psychopharmacol. 2007; 21(8): 833-6. [ Links ]
8. Zill P, Baghai TC, Zwanzger P, Schüle C, Eser D, Rupprecht R, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004; 9(11): 1030-6. [ Links ]
9. Hung CF, Lung FW, Hung TH, Chong MY, Wu CK, Wen JK, et al. Monoamine oxidase A gene polymorphism and suicide: an association study and meta-analysis. J Affect Disord. 2012; 136(3): 643-9. [ Links ]
10. Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999; 21(2 Suppl): 99S-105S. [ Links ]
11. Grahame-Smith DG. Tryptophan hydroxylation in brain. Biochem Biophys Res Commun 1964; 16(6): 586-92. [ Links ]
12. Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003; 100(23): 13525-30. [ Links ]
13. Invernizzi RW. Role of TPH-2 in brain function: news from behavioral and pharmacologic studies. J Neurosci Res. 2007;85(14):3030-5. [ Links ]
14. Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003; 299(5603): 76. [ Links ]
15. Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004; 305(5681): 217. [ Links ]
16. Chen GL, Novak MA, Hakim S, Xie Z, Miller GM. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: association with hypothalamic-pituitary-adrenal axis function and in vitro gene expression. Molecular psychiatry 2006; 11(10): 914-28. [ Links ]
17. Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005; 45(1): 11-6. [ Links ]
18. Hong KW, Sugawara Y, Hasegawa H, Hayasaka I, Hashimoto R, Ito S, et al. A new gain-of-function allele in chimpanzee tryptophan hydroxylase 2 and the comparison of its enzyme activity with that in humans and rats. Neurosci Lett. 2007; 412(3): 195-200. [ Links ]
19. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997; 349(9063): 1436-42. [ Links ]
20. Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, et al. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005; 62(10): 1109-18. [ Links ]
21. Tsai SJ, Hong CJ, Liou YJ, Yu YW, Chen TJ, Hou SJ, et al. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33(4): 637-41. [ Links ]
22. Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003; 25(2): 115-21. [ Links ]
23. Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005; 15(2): 97-8. [ Links ]
24. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2): 175-91. [ Links ]
25. Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, et al. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998; 44(11): 1099-117. [ Links ]
26. Mushtaq R, Shoib S, Shah T, Mushtaq S. Tryptophan hydroxylase 2 gene polymorphism in anxiety and depressive disorder in kashmiri population. Journal of clinical and diagnostic research: JCDR. 2014; 8(6): WC01-3. [ Links ]
27. Wang X, Wang Z, Wu Y, Hou Z, Yuan Y, Hou G. Tryptophan hydroxylase 2 gene is associated with cognition in late-onset depression in a Chinese Han population. Neurosci Lett. 2015;600: 98-103. [ Links ]
28. Zhang Y, Chang Z, Chen J, Ling Y, Liu X, Feng Z, et al. Methylation of the tryptophan hydroxylase2 gene is associated with mRNA expression in patients with major depression with suicide attempts. Mol Med Rep. 2015;12(2): 3184-90. [ Links ]
29. Smith BW, Zautra AJ. The effects of anxiety and depression on weekly pain in women with arthritis. Pain. 2008; 138(2): 354-61. [ Links ]
30. Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord. 2003; 74(1): 85-96. [ Links ]
31. Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB. Individual differences in changes in mood and platelet monoamine oxidase (MAO) activity during hormonal replacement therapy in menopausal women. Psychoneuroendocrinology. 1996; 21(7): 575-92. [ Links ]
32. Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacological reviews. 1992; 44(3): 401-58. [ Links ]
33. Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Mol Neurobiol. 1998; 18(2): 87-123. [ Links ]
34. Fan R, Knapp M. Genome association studies of complex diseases by case-control designs. Am J Hum Genet. 2003; 72(4): 850-68. [ Links ]
35. Lim JE, Pinsonneault J, Sadee W, Saffen D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Mol Psychiatry. 2007; 12(5): 491-501. [ Links ]
36. Pagenstecher C, Wehner M, Friedl W, Rahner N, Aretz S, Friedrichs N, et al. Aberrant splicing in MLH1 and MSH2 due to exonic and intronic variants. Hum Genet. 2006; 119(1-2): 9-22. [ Links ]
37. Cartegni L, Hastings ML, Calarco JA, de Stanchina E, Krainer AR. Determinants of exon 7 splicing in the spinal muscular atrophy genes, SMN1 and SMN2. Am J Hum Genet. 2006; 78(1): 63-77. [ Links ]
38. Pagani F, Baralle FE. Genomic variants in exons and introns: identifying the splicing spoilers. Nat Rev Genet. 2004; 5(5): 389-96. [ Links ]
39. Pagani F, Stuani C, Tzetis M, Kanavakis E, Efthymiadou A, Doudounakis S, et al. New type of disease causing mutations: the example of the composite exonic regulatory elements of splicing in CFTR exon 12. Hum Mol Genet. 2003; 12(10): 1111-20. [ Links ]
![]() Correspondence:
Correspondence:
Jing Du and Yongyong Shi
E-mail address: dujing42@126.com (Jing Du)
shiyongyong3@gmail.com (Yongyong Shi)
Received: 3 July 2015
Revised: 5 January 2016
Accepted: 19 January 2016