Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.7 nov. 2007
Factors related to survival from oral cancer in an Andalusian population sample (Spain)
Manuel Vallecillo Capilla, Maria Nuria Romero Olid, Maria Victoria Olmedo Gaya, Candela Reyes Botella, Vicente Bustos Ruiz
Cirugía Oral. Facultad de odontología. Universidad de Granada. Departamento de Cirugía Oral y Maxilofacial. Hospital Universitario "Virgen de las Nieves" de Granada
ABSTRACT
Introduction: Approximately 3% of malignant tumors originate in the oral cavity. The majority are squamous cell carcinomas, and a small percentage, malignant tumors of the salivary glands, lymphoreticular diseases, bone tumors, melanomas, sarcomas, malignant odontogenic tumors and metastases of tumors from other locations. The prognosis of these pathologies depends on the size, infiltration, and site of the lesion, the presence or absence of metastatic spread, and to a certain degree the differentiation of the tumor. The prognosis of an oral cancer remains generally negative, with 5-year survival figures below 50%, producing high rates of mortality and morbidity.
Objectives: To evaluate the influence of different variables on survival in an oral cancer population.
Patients and methods: Two-hundred and sixteen patients with oral squamous cell carcinoma were studied over a period of five years, evaluating 42 variables grouped into five data sections: personal, lesion, site, stage, and risk factors.
Results and conclusions: Average survival was 2088 days, with a standard deviation of 98 days. The factors most associated with mortality were: location in the gingiva (p=0.0590), in the trigone (p=0.0104), size (T3-T4) (p=0.0004) and lymph node involvement (N2a-N2b) (p=0.0035). Tobacco and alcohol, nowadays considered to be highly significant in carcinogenesis, had no considerable influence on survival.
Key words: Oral cancer survival, mortality and risk factors.
RESUMEN
Introducción: aproximadamente un 3% de los tumores malignos se origina en la cavidad oral. La mayoría corresponden a carcinomas de células escamosas, y un pequeño porcentaje a tumores malignos de glándulas salivares, enfermedades linforeticulares, tumores óseos, melanomas, sarcomas, tumores malignos odontogénicos y metástasis de tumores de otras localizaciones. El pronóstico de estas patologías depende del tamaño, infiltración y localización de la lesión, presencia o ausencia de extensión metastática y en cierto grado de la diferenciación del tumor1. La patología neoplásica oral aún mantiene un pronóstico general negativo, con unas cifras globales de supervivencia a los 5 años menores del 50%, produciendo altas tasa de mortalidad y morbilidad2,3.
Objetivo: evaluar la carga de distintas variables sobre el tiempo de supervivencia en una población con cáncer oral.
Pacientes y metodo: sobre 216 paciente realizamos un estudio de casos de cáncer de oral de células escamosas a 5 años, evaluando 42 variables, agrupadas en cinco apartados: datos personales, de la lesión, localización, estadiaje y factores de riesgo.
Resultados y conclusiones: el tiempo medio de supervivencia fue 2088 días, con un error estándar de 98 días. Los factores asociados a mayor mortalidad fueron: localización en la encía (p= 0.0590), en trígono (p= 0.0104), el tamaño (T3-T4) (p= 0.0004) y afectación ganglionar (N2a-N2b) (p= 0.0035). El tabaco y el alcohol, considerados hoy de gran importancia en la génesis del cáncer, no influyeron considerablemente en el tiempo de supervivencia.
Palabras clave: cáncer oral, tiempo de supervivencia, mortalidad y factores de riesgo.
Introduction
Cancer is a multifactorial disease, brought on by a combination of causal and predisposing factors, and which at a given moment and under favourable conditions may take effect in predisposed persons. Mortality from malignant neoplasms figures amongst the principal causes of death worldwide, and is therefore a highly serious public health problem. The primary incidences of cancers among men are of the prostate, lung and bronchus, and colon and rectum; and in women particularly breast, lung and bronchus, colon and rectum (4). Approximately 3% of malignant tumors originate in the oral cavity. The majority correspond to squamous cell carcinomas, and a small percentage to malignant tumors of the salivary glands, lymphoreticular disease, bone tumors, melanomas, sarcomas, malignant odontogenic tumors and oral metastases of tumors from other locations. The prognosis of these pathologies depends on the size, infiltration and site of the lesion, presence or absence of metastasis and to a certain degree the differentiation of the tumor (1). Oral cancer still has a generally negative prognosis, with five-year survival figures of less than 50%, producing high rates of mortality and morbidity (2, 3). There is a wide variation in the incidence and mortality rates of oral cancer in the different regions throughout the world. The incidence of oropharyngeal and oral cancer in men is greater in the Lower Rhine area of France; in the south of India, where it is the most frequent form of cancer, in certain areas of central and eastern Europe, and in some regions of Latin America (5, 6). In females the highest incidence is found in India, with a moderate increase in mortality in Central and Eastern Europe observed during the eighties and nineties (7). Cohort studies demonstrate that the incidence of oral cancer has increased in all ethnic groups worldwide in recent decades (6), especially in young men in Eastern Europe (7, 8). The increased risk has been noted in 19 out of 24 European countries, finding an increase of 3 to 10 times within a single generation (9, 10); however, a tendency for the incidence of oral cancer to reduce in certain countries of Latin America and the Caribbean has been observed (5). Thus, oral cancer is a not very frequent disease, with an incidence that varies according to the geographic area, these epidemiological differences being largely attributable to the different customs prevailing in each region, above all with respect to the prevalence of known risk factors.
When reviewing the literature, we can see how different factors have been evaluated as prognostic markers in oral cancer. Leite et al. (11) studied various parameters, highlighting clinical stage, gender, early diagnosis, treatment modality and the time elapsed between initial symptoms and treatment as principal prognostic factors. Beltrami et al. (12), studied the prognostic influence of factors such as tumor site, size, microscopic grade and DNA content. Other authors, such as González et al. (13), evaluated clinical and histopathological parameters in relation to survival, the most influential factors being location, size, lymph node metastasis, clinical stage, degree of cellular differentiation and pleomorphism.
In a study carried out at the Canniesburn Hospital, Glasgow in 2000, the authors concluded that despite the advances in diagnosis and adjuvant treatment, no improvement in survival has been seen in the last 16 years, referring to a five-year survival rate of 44% (14, 15).
Given that few studies have been carried out on prognostic indicators for survival in oral cancer, and that in many of these studies a multivariate analysis was not used to justify the results, the objective of our study is to evaluate the influence of different variables on survival in an oral cancer population.
Patients and methods
The study population comprised all patients diagnosed with oral squamous cell carcinoma at the Oral and Maxillofacial Surgery Unit, Granada, between January 1994 and May 1999. The clinical records were compiled at the Maxillofacial Surgery Unit, from the surgical archives of the Traumatology Hospital, the Central Archive of the University Hospital, Virgen de las Nieves, and the database of the NHI (National Health Institute).
It was possible to select and review 253 case histories: 37 were rejected for having an incomplete clinical history, lack of data regarding diagnostic tests, for incomplete data on clinical course and evolution, transfer to another hospital, or for lack of continuity in follow-up. The sample therefore reduced to 216.
Patients were aged between 21 and 96 years, with the median situated in the 60-70 decade. The minimum age was 21 and the maximum 96, giving a range of 75 years; however the next highest age above 21 was 29, which would indicate that the first case was exceptional. With regard to gender, 75.9% were male and 24.1% female.
The variables included in the study were: origin (rural/urban), province (Almeria, Granada, Jaen), gender, age, presence of neoplastic lesions, tumor site, size, lymph node involvement, smoking, consumption of alcohol, periodontal disease, prosthetic trauma, trauma caused by sharp teeth and edentulism.
The study design was observational, analytical and retrospective. The data sheet for data collection in the study group contained 42 variables, grouped into five sections: personal data, lesions, location, preoperative stage or characteristics of the tumor, and risk factors.
Survival for each variable was analyzed using the Kaplan Meier method, and comparison between variables made using the Breslow test. Finally the multivariate analysis to obtain independent predictors of mortality was made using Cox proportional hazards models. Construction of the final model containing those variables found to be independently associated with survival in oral cancer was made in three stages: in the first, adjustments were made for each variable separately plus those that were clearly significant, then a new model was adjusted in which the combined effect of these variables was measured (Model I). From this model, those variables that could be eliminated without loss of information were determined; thus creating the definitive model, which we called Model II.
Results
Data relating to death were compiled through the NHI. Of the 216 patients, the current situation is known for 188, while certain information for the remainder was lacking and could therefore not be used. Of the 188 patients, 44 died from the disease.
From the survival distribution (Figure 1), we calculated the possible parameters, thus the mean survival was 2088 days with a standard deviation of 98 days; the median could not be calculated since of the 188 patients, only 44 died corresponding to 23.4% of deceased patients and not the 50% required to calculate the median. In any case, the 25 percentile was 915 days with a standard deviation of 2111 days, indicating that 75% of patients survived for more than 915 days.
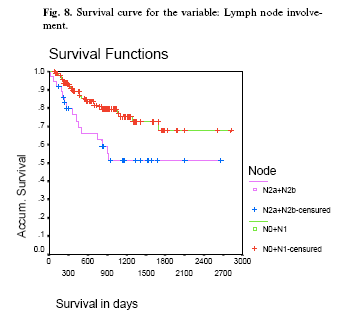
After carrying out a bivariate correlation for the survival curves of the different factors studied, the most relevant factors are as follows: Regarding province, there were no significant differences (X2exp= 2.08, 2g.1., p= 0.273), however, the curve for Almeria did seem somewhat better, although without reaching significance. On examining gender, there were indications that men with oral cancer tend to survive for shorter periods than women (X2exp= 3.73, 1g.1., p= 0.0535) (Figure 2). There are indications that patients with previous neoplastic lesions tend to survive longer than those without, (X2exp= 3.54, 1g.1., p= 0.0599) (Figure 3). With respect to site, the results were as follows: discreet, almost significant differences among those were the lesion presented in the gingiva, who appear to have shorter survival (X2exp= 3.57, 1g.1., p= 0.0590) (Figure 4). Patients with a lesion in the labial commissures also tend to have shorter survival (X2exp= 3.71, 1g.1., p= 0.0542); however, it must be taken into account that the sample size was small, 5 out of 188, and this result should be taken with caution (Figure 5). Patients with lesions in the retromolar trigone also showed a reduced survival (X2exp= 6.57, 1.g1., p= 0.0104). The average survival in the 165 patients without a lesion in this area was 2184 days, against 926 days for the 27 patients who did have a lesion in the trigone (Figure 6). On the tongue, 101 lesions were found, 7 in the ventral tongue, none on the dorsal tongue, 10 on the anterior left lateral tongue, 16 on the mid left lateral tongue, 13 on the posterior left tongue, 4 on the anterior right lateral tongue, 22 on the mid right lateral tongue, 5 on the posterior third right lateral tongue, 16 on the left base of the tongue, and 8 on the right base of the tongue. There were no significant differences between the survival curves for the different tongue sites (X2exp= 6.9, 1.g1., p= 0.4064).
Regarding tumor characteristics, this information was lost in some patients, finding that 61 patients who presented the tumor in stages T3-T4 had a lower survival than the 124 in stages T1-T2. While the mean survival in the first group was 1576 days, in the second this was 2265 days (X2exp= 12.66, 1g.1., p= 0.0004) (Figure 7). Those patients with tumors in stages N2a-N2b, had a lower survival than those who were in stages N0-N1 (X2exp= 8.51, 1g.1., p= 0.0035) (Figure 8). With respect to smoking, no significant differences were found between the survival curves for this variable (X2exp= 1.96, 1g.1., p= 0.1620). This could be due to the strong imbalance between the sample sizes: while 144 were smokers, only 48 when non-smokers (Figure 9). Although there are no significant differences, (X2exp= 2.47, 2.g.1., p= 0.1933), it would seem clear that those patients with slight periodontal disease lived on average longer than those with serious periodontal disease or edentulous patients (Figure 10).
The remainder of the variables studied presented no significant differences between their survival curves.
The multivariate analysis (Table 1) was made using those variables found to be significant in the bivariate analysis, thus determining the influence on survival of each variable independently, since for model 0, the results obtained may have been due to a certain influence of some variables on others.
From the multivariate analysis Model I was obtained in which it could be observed that the majority of the variables reduced in significance; in addition in the case of the commissure, tobacco and alcohol had clearly lost significance, thus a new model was tried in which these variables were eliminated, observing that no information was lost, thus leaving us with Model II as the definitive. From this model we can conclude that presenting a lesion in the gingiva increases risk of death by 1.72 times than when it does not, and that the presence of a lesion in the trigone increases the risk of death by 2.14 times than were the lesion not at this site. Patients with tumors of size T3-T4 present a risk of death 1.89 times greater than those with tumors in lower stages. Patients whose tumor was in stage N2a-N2b had a risk of death of 1.60 times higher than those with tumors in lower stages.
Discussion
In the results section, we observed mean survival figures of 2088 days, and more than 915 days for 75% of patients, other studies offer figures of 43% at five years, Mohit-Tabatabay et al. (16) and Al-Kourainy (17), we can also observe in Table 1 how risk of death from oral cancer is increased by 1.7 times more in smokers than in non-smokers, a small percentage by any standards, given the importance attributed to this risk factor in the majority of studies. We should take into account that the disproportion between the sample sizes does not allow us to extract net conclusions with respect to the role played by tobacco.
With regard to survival from oral cancer between rural and urban populations, it can be seen from the group of cases that patients from rural areas have lower survival with respect to those from urban areas, a possible hypothesis being a worse access to diagnosis and treatment, likewise the possibly lower sociocultural level, continued exposure to the sun and dietary habits.
Different opinions exist in the literature with regard to gender as a possible prognostic factor. Pugliano et al. (18) speak of a greater survival in women; however Shah et al. (19) believe that survival in females is lower. Other authors find no significant differences between the sexes. In our study, we can see indications that males tend to survive less, however without being clearly significant.
It is important to determine the influence the site of the primary tumor has on the prognosis for the patient. Among the reasons given in explanation of this difference in prognosis are found ease of early diagnosis, accessibility or ease for extirpation of the tumor with sufficient surgical margin, and the different lymphopathy that each site presents, and which manifests in its greater metastatic capacity.
Our study indicates that the location of oral squamous cell carcinoma in the gingiva and in the trigone carries the greatest risk of mortality, while others such as the palate, tongue (at the different sites) or floor of the mouth do not appear to influence survival. These results appear to disagree with other studies in which the tongue is the site that demonstrates the lowest survival period (20-23).
It is also important to comment that location in the labial commissure could be a variable to take into account in survival, since the mean life-expectancy is much lower in those patients who present a lesion in this site than those that do not, however we were obliged to eliminate this factor from the definitive model due to the reduced sample size.
The size of the primary tumor has always been considered as a fundamental factor in the majority of tumor prognosis and staging systems, including the most universal (TMN), in which one of its pillars, the status of regional lymph nodes, constitutes a highly representative prognostic factor (3). Thus, Kalnins et al. (24) affirm that the presence of lymphadenopathy reduces survival at five years to 45%. With regard to these factors, our results are in agreement, relating the higher T and N stages with lower overall survival.
On the other hand, although in our study we find that smoking increases the risk of death from oral cancer, this is by only a small percentage given the importance attributed to this factor in the majority of publications (13, 16). This variable even loses significance on carrying out the multivariate analysis for which reason it was eliminated as a prognostic factor in overall survival. However, we are in agreement with other authors such as Browman et al. (25) who demonstrated that those cancer patients who continue smoking during radiotherapy had a lower survival than others. Silverman et al. (26) observed a decrease in risk of a second oral or oropharyngeal primary cancer among those patients who reduced their smoking habit, which would seem to confirm that smoking has a greater affect on a previously altered mucosa.
Regarding alcohol consumption, as occurred with smoking, on carrying out the multivariate analysis this variable lost significance, which would indicate that its effect is influenced by other variables. Thus, Bundgaard et al. (27) eliminate alcohol as an independent prognostic factor, since the impact of alcohol on survival could be related to the close relationship between alcohol consumption and smoking.
Conclusions
There are indications that patients with previous lesions tend to survive for longer than those without; presenting the lesion in the gingiva increases risk of death by 1.72 times than when it is not in this site; location of the lesion in the trigone increases risk of death by 2.14 times against those not in this site; patients with tumor size greater than T3-T4 had a risk of death 1.89 times higher than those with a tumor in lower stages; patients with a tumor at N2a-N2b have a risk of death 1.6 times higher than those with a tumor at lower stages.
References
1. Esteban F, Gonzalez-Moles MA, Ruiz-Avila I. Pathological malignancy grading and prognosis of head and neck cancer. Med Oral. 1998 May;3(3):148-162 [ Links ]
2. Martinez-Conde R, Aguirre JM, Burgos JJ, Rivera JM. Clinicopathological factors in early squamous cell carcinoma of the tongue and floor of the mouth, in Biscay (the Basque Country, Spain). Med Oral. 2001 Mar-Apr;6(2):87-94. [ Links ]
3. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002 Jul-Aug;52(4):195-215 [ Links ]
4. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan-Feb;57(1):43-66. [ Links ]
5. Franceschi S, Bidoli E, Herrero R, Munoz N. Comparison of cancers of the oral cavity and pharynx worldwide: etiological clues. Oral Oncol. 2000 Jan;36(1):106-15. [ Links ]
6. Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of mouth cancer: a review of global incidence. Oral Dis. 2000 Mar;6(2):65-74. [ Links ]
7. La Vecchia C, Lucchini F, Negri E, Levi F. Trends in oral cancer mortality in Europe. Oral Oncol. 2004 Apr;40(4):433-9. [ Links ]
8. La Vecchia C, Tavani A, Franceschi S, Levi F, Corrao G, Negri E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997 Sep;33(5):302-12. [ Links ]
9. Macfarlane GJ, Boyle P, Evstifeeva TV, Robertson C, Scully C. Rising trends of oral cancer mortality among males worldwide: the return of an old public health problem. Cancer Causes Control. 1994 May;5(3):259-65. [ Links ]
10. Robinson KL, Macfarlane GJ. Oropharyngeal cancer incidence and mortality in Scotland: are rates still increasing. Oral Oncol. 2003 Jan;39(1):31-6. [ Links ]
11. Leite IC, Koifman S. Survival analysis in a sample of oral cancer patients at a reference hospital in Rio de Janeiro, Brazil. Oral Oncol. 1998 Sep;34(5):347-52 [ Links ]
12. Beltrami CA, Desinan L, Rubini C. Prognostic factors in squamous cell carcinoma of the oral cavity. A retrospective study of 80 cases. Pathol Res Pract. 1992 Jun;188(4-5):510-6. [ Links ]
13. González Moles M, Rodríguez Archilla A, Caballero R, Ruiz Ávila I, Garcia Anillo M, Bravo I. Estudio de los parámetros clínicos e histopatológicos del carcinoma epidermoide de cavidad oral. Implicaciones pronósticas. Avances en Odontoestomatología 1998; 14: 589-610. [ Links ]
14. Haddadin KJ, Soutar DS, Webster MH, Robertson AG, Oliver RJ, MacDonald DG. Natural history and patterns of recurrence of tongue tumours. Br J Plast Surg. 2000 Jun;53(4):279-85. [ Links ]
15. Jones AS. Prognosis in mouth cancer: tumour factors. Eur J Cancer B Oral Oncol. 1994 Jan;30B(1):8-15. [ Links ]
16. Mohit-Tabatabai MA, Hill GJ, Raina S, Rush BF, Ohanian M. Multimodality preoperative treatment for advanced stage IV (MO) cancer of the head and neck. Am J Surg. 1990 Oct;160(4):370-2. [ Links ]
17. Al-Kourainy K, Kish J, Ensley J, Tapazoglou E, Jacobs J, Weaver A, Crissman J, Cummings G, Al-Sarraf M. Achievement of superior survival for histologically negative versus histologically positive clinically complete responders to cisplatin combination in patients with locally advanced head and neck cancer. Cancer. 1987 Jan 15;59(2):233-8. [ Links ]
18. Pugliano FA, Piccirillo JF, Zequeira MR, Fredrickson JM, Perez CA, Simpson JR. Clinical-severity staging system for oral cavity cancer: five-year survival rates. Otolaryngol Head Neck Surg. 1999 Jan;120(1):38-45. [ Links ]
19. Shah JP, Cendon RA, Farr HW, Strong EW. Carcinoma of the oral cavity. factors affecting treatment failure at the primary site and neck. Am J Surg. 1976 Oct;132(4):504-7. [ Links ]
20. Morelatto RA, Lopez de Blanc SA. Oral cancer mortality in the province of Cordoba, Argentine Republic in the period 1975-2000. A comparative study with other populations. Med Oral Patol Oral Cir Bucal. 2006 May 1;11(3):E230-5. [ Links ]
21. Shiboski CH, Shiboski SC, Silverman S Jr. Trends in oral cancer rates in the United States, 1973-1996. Community Dent Oral Epidemiol. 2000 Aug;28(4):249-56. [ Links ]
22. Nieto A, Ramos MR. Rising trends in oral cancer mortality in Spain, 1975-94. J Oral Pathol Med. 2002 Mar;31(3):147-52. [ Links ]
23. Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of tongue cancer: a review of global incidence. Oral Dis. 2000 Mar;6(2):75-84. [ Links ]
24. Kalnins IK, Leonard AG, Sako K, Razack MS, Shedd DP. Correlation between prognosis and degree of lymph node involvement in carcinoma of the oral cavity. Am J Surg. 1977 Oct;134(4):450-4. [ Links ]
25. Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, Skingley P, Levine MN. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993 Jan 21;328(3):159-63. [ Links ]
26. Silverman S Jr, Gorsky M, Greenspan D. Tobacco usage in patients with head and neck carcinomas: a follow-up study on habit changes and second primary oral/oropharyngeal cancers. J Am Dent Assoc. 1983 Jan;106(1):33-5 [ Links ]
27. Bundgaard T, Bentzen SM, Wildt J. The prognostic effect of tobacco and alcohol consumption in intra-oral squamous cell carcinoma. Eur J Cancer B Oral Oncol. 1994 Sep;30B(5):323-8. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Manuel Vallecillo Capilla
Cirugía Oral. Facultad de odontología. Universidad de Granada.
Dpto de Cirugía Oral y Maxilofacial.
Hospital Universitario"Virgen de las Nieves" de Granada.
Campus de Cartuja s/n. 18071-GRANADA. Spain
E-mail: mvalleci@ugr.es
Received: 30-10-2005
Accepted: 16-09-2007