INTRODUCTION
Clinically applied pharmacogenetics is a promising tool for delivering individualized pharmacotherapy.1, 2 Researchers and clinicians theorize that implementation of pharmacogenetic testing (genotyping) will contribute meaningfully to precision medicine through selection of drugs that are specifically targeted for individual patients based on underlying genetic variation.3 Variants in CYP2C19, the gene that codes for the cytochrome P450 (CYP) 2C19 drug metabolizing enzyme, have been associated with variable CYP2C19 metabolic activity. While CYP2C19*2 -*8 polymorphisms are loss-of-function alleles that decrease metabolism, CYP2C19*17 is an increased function allele that increases metabolism.4 CYP2C19*2 and CYP2C19*17 are common in major race/ethnic groups. CYP2C19*2 is present in 15% of Caucasians, 18% of African Americans, and 29-34% of Asians.5 CYP2C19*17 is present in 22% of Caucasians, 19% of African Americans, and 2-17% of Asians.5 Despite existing data associating variant CYP2C19 genotypes with altered metabolizing enzyme phenotypes, clinical implementation of genotyping is not routine. The dearth of routine genotyping may be due to barriers such as cost of genetic testing, delayed availability of test results, lack of supportive infrastructure (e.g., clinical decision support tools within electronic health records), educational gaps in knowledge of testing and interpretation of results among front-line clinicians, and in the case of clopidogrel in particular, lack of endorsement by the American College of Cardiology/American Heart Association citing the absence of published randomized controlled trials demonstrating that genotyping improves clinical outcomes.6,7,8,9,10,11,12
Patients presenting with acute coronary syndromes (ACS) require expeditious antiplatelet therapy, consisting of aspirin plus a P2Y12 inhibitor, regardless of the management approach (e.g., interventional vs. medical management).13,14,15 Clopidogrel, a pro-drug whose effectiveness depends on in vivo biotransformation to an active metabolite by CYP2C19, has long been the P2Y12 inhibitor agent of choice in this setting. Compared with normal metabolizers (i.e., CYP2C19*1/*1), patients with loss-of-function alleles (i.e., CYP2C19*2 -*8) are more likely to be hypo-responsive to clopidogrel due to decreased inhibition of platelet aggregation and at an increased risk for major adverse cardiovascular events.16,17 Recent drug approvals for prasugrel and ticagrelor make available alternative agents whose effectiveness is less susceptible to genetic variation in CYP2C19 than is clopidogrel.18,19,20,21,22,23
There is some evidence to support clinical implementation of CYP2C19 genotyping in the acute care setting. Preliminary data from the Implementing Genomics in Practice (IGNITE) Pharmacogenetics Working Group Investigators demonstrated that subjects with a loss-of-function CYP2C19 genotype who received clopidogrel had more than double the rate of major adverse cardiac events at 12 months compared with those who received a clopidogrel alternative (i.e., prasugrel or ticagrelor) after percutaneous coronary intervention (PCI).24 Additionally, the RAPID GENE Study evaluated the feasibility of inpatient, pre-procedural (‘just-in-time’) CYP2C19 genotyping.25 This study reported that no patients who received “just-in-time” genotyping with results guiding initial P2Y12 inhibitor therapy choice experienced high residual platelet function, while 30% of patients in the standard of care group did. The study test was able to deliver results in approximately 1-2 hours and was 99.4% accurate. In general, evidence is limited regarding clinical implementation of CYP2C19 genotyping in ambulatory care settings; most studies included patients based on prescriptions for clopidogrel rather than their indication for clopidogrel, and provider acceptance of genotype-based pharmacotherapy recommendations was not routinely reported.26,27
Given these gaps in knowledge, the objective of this study was to assess the feasibility of clinical pharmacist-provided CYP2C19 genotype-guided P2Y12 inhibitor therapy recommendations to cardiologists at an outpatient cardiology practice. The rationale for our study was twofold: 1) to develop an understanding of the feasibility of clinical pharmacogenetic testing by clinical pharmacists in an ambulatory clinic setting; and, 2) to expand the understanding of evaluating CYP2C19 genotyping in non-traditional settings (e.g., integrated care delivery system).
METHODS
Study design. This was a prospective, open-labeled, single-arm study conducted at Kaiser Permanente Colorado (KPCO), an integrated healthcare delivery system. At the time of the study, KPCO provided care to more than 530,000 patients in Colorado at 18 medical offices with cardiology specialty services provided by 22 cardiologists at two of these clinics. At KPCO, clinical pharmacists are integrated within medical office practices and work collaboratively with physicians and other providers to provide direct patient care, focused drug therapy expertise, and disease state management.
Study participants. Eligible patients were age 18 years or older who were referred for non-emergent (i.e., elective) cardiac catheterization between March 1, 2013 and January 23, 2014. Patients had continuous KPCO coverage during the 180 days prior to and 90 days following enrollment screening, were followed by a KPCO cardiologist, and had not received a P2Y12 inhibitor within two weeks preceding enrollment. Patients were excluded if they: (1) had a documented history of cerebrovascular accident or transient ischemic attack; (2) had a major bleeding episode requiring intervention or hospitalization within the year prior to enrollment screening; (3) were receiving concomitant anticoagulant therapy (e.g., warfarin, dabigatran, rivaroxaban, low molecular weight heparin, unfractionated heparin) at the time of enrollment screening; (4) had a hemoglobin measurement <10 g/dL within the three months prior to enrollment screening; (5) had a history of end stage renal disease; (6) were pregnant or nursing at time of enrollment screening; (7) had a known allergy or history of hypersensitivity reaction to aspirin, clopidogrel, prasugrel, or ticagrelor; (8) were > 75 years old at time of enrollment screening; (9) were diagnosed with ACS within 30 days prior to enrollment screening; (10) were decisionally-impaired; or (11) were unable or unwilling to provide written, informed consent. The KPCO Institutional Review Board reviewed and approved all study activities.
Patient recruitment. Patients were recruited for the study based on cardiologist referral for non-emergent cardiac catheterization. Potential patients were identified during office consultation with their cardiologist. Upon completion of the clinical evaluation of the patient where cardiac catheterization was recommended, cardiologists referred patients to a clinical pharmacist for enrollment screening. The clinical pharmacist conducted a review of the electronic health record (EHR) to determine whether the patient was eligible for study participation. The clinical pharmacist met with eligible patients in their cardiologist’s office to discuss implications of pharmacogenetic testing and describe the purpose of the study. If the patient had already left the clinic, s/he received a telephone call to discuss implications of pharmacogenetic testing, describe the purpose of the study, and if the patient was interested in the study, arrange to meet in-person with the clinical pharmacist at a KPCO clinic location convenient for the patient. Interested patients were asked to provide written informed consent. Patients who provided consent were asked to donate a buccal swab for CYP2C19 genotyping. Cardiologists were not consented as their role in the study was considered a part of routine care. Patient recruitment activities were designed to continue until at least 10 patients consented to genotyping and results were available and reported.
Study intervention. For patients who provided informed consent, the clinical pharmacist provided the patient with a buccal swab DNA collection kit (Genelex Laboratory, Seattle, WA). After obtaining, labeling, and packaging the sample, the clinical pharmacist mailed the package overnight to Genelex for CYP2C19 genetic analysis. After genotyping, Genelex emailed a secure PDF file containing the results to the clinical pharmacist. The results were then sent to KPCO’s Lab Client Services for electronic posting in the patient’s EHR. Once CYP2C19 genotype results were available, the clinical pharmacist assessed the results and provided recommendations to the cardiologist for individualized antiplatelet therapy based upon CYP2C19 genotype in accordance with evidence-based clinical practice guidelines (Table 1).28
Table 1 CYP2C19 Genotype Results, Antiplatelet Therapy Recommendations, and Physician Response for N=6 Patients
| CYP2C19 Genotype | CYP2C19 Phenotypea | Number (%) Patients | Antiplatelet Therapy Recommendation | Physician Response |
|---|---|---|---|---|
| *1/*1 | Normal metabolizer (fully functional enzyme activity) | 3 (50%) | Clopidogrel 75mg daily | Approved (100%) |
| *1/*2 | Intermediate metabolizer (decreased enzyme activity) | 1 (17%) | Ticagrelor 90 mg twice daily | Approved (100%) |
| *1/*17 | Rapid metabolizer (increased enzyme activity compared to normal metabolizers) | 1 (17%) | Clopidogrel 75mg daily | Approved (100%) |
| *2/*17b | Intermediate metabolizer (decreased enzyme activity) | 1 (17%) | Clopidogrel 75mg daily | Approved (100%) |
aCYP2C19 phenotypes are based on consensus terms outlined in reference 4.
bAlthough the current 2013 Clinical Pharmacogenetics Implementation Consortium (CPIC) recommends using an alternative to clopidogrel for CYP2C19*2/*17 (outlined in reference 28), the 2011 CPIC guidelines available at the time the study was conducted did not discuss the CYP2C19*2/*17 genotype nor did the guidelines provide recommendations to utilize alternative therapy. Therefore, the clinical pharmacist recommended clopidogrel for this patient.
Data collection. Patient samples were genotyped for the following single nucleotide polymorphisms in the CYP2C19 gene: CYP2C19*2 (c.681G>A; rs4244285), CYP2C19*3 (c.636G>A; rs4986893), CYP2C19*4 (c.1A>G; rs28399504), CYP2C19*5 (c.1297C>T; rs56337013), CYP2C19*6 (c.395G>A; rs72552267), CYP2C19*7 (c.819+2T>A; rs72558186), CYP2C19*8 (c.358T>C; rs41291556), and CYP2C19*17 (c.-806 C>T, rs12248560). CYP2C19*2 -*8 are loss-of-function alleles, while CYP2C19*17 is an increased function allele. Absence of these polymorphic alleles indicated a CYP2C19*1/*1 (wild-type) genotype. CYP2C19*4 -*8 alleles are rare (allele frequency < 1% in the general population) and were not expected to be found in our study population.5 Nonetheless, since these are also loss-of-function alleles, failing to test for them would be associated with a small risk of false negative results. Genotyping was performed using a previously developed and validated genotyping platform for CYP2C19 (Genelex, Seattle, WA), that was fully Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) compliant. Turnaround time for genotyping results ranged between 3- 5 business days. Patients were phenotypically classified as CYP2C19 ultra-rapid, rapid, normal, intermediate, or poor metabolizers based on the presence of CYP2C19*1 (wild-type), *2-*8, and *17 alleles, consistent with current guidelines.4
Outcome and recommendation. P2Y12 inhibitor therapy recommendations were developed and documented using a consultation template supported by published clinical practice guidelines for clinical action based upon CYP2C19 genotype.28 The use of CYP2C19 genotype to inform clopidogrel therapy is a Clinical Pharmacogenetics Implementation Consortium Level A classification defined as “genetic information should be used to change prescribing of affected drug”.29 In addition, the FDA-approved drug label for clopidogrel states that CYP2C19 poor metabolizers may experience diminished antiplatelet effect of the drug and an alternative P2Y12 inhibitor should be considered.30 For this study, P2Y12 inhibitor recommendations were provided to the cardiologist via EHR messaging (standard communication preference by KPCO cardiologist), and the cardiologist then indicated approval or non-approval. CYP2C19 genotyping results and cardiologist recommendations were then communicated to the patient by the clinical pharmacist via telephone and letter.
Data analysis. Analyses for this study were descriptive. CYP2C19 genotyping results (e.g., frequency of specific genotypes/phenotypes within the study population) and P2Y12 inhibitor recommendations provided by the clinical pharmacists were collected and reported. Additionally, the percentages of patients who consented to and completed genotyping, patients for whom pharmacogenetic test results were available prior to cardiac catheterization, and recommendations accepted by cardiologists are reported.
RESULTS
Cardiologist consultation and referral yielded 43 patients who were scheduled for non-emergent cardiac catheterization during the study period. Of the 22 patients who met study inclusion criteria and were contacted for recruitment, six patients (27%) were consented and genotyped (Figure 1). Genotyping results were available for all of study patients prior to cardiac catheterization. Clinical pharmacists’ P2Y12 inhibitor recommendations were accepted by the cardiologists for all of the study patients (Figure 2). The study population had a mean age of 64 years and was two-thirds (67%) male. Fifty percent of patients were Caucasian, 33% Hispanic, and 17% African-American.
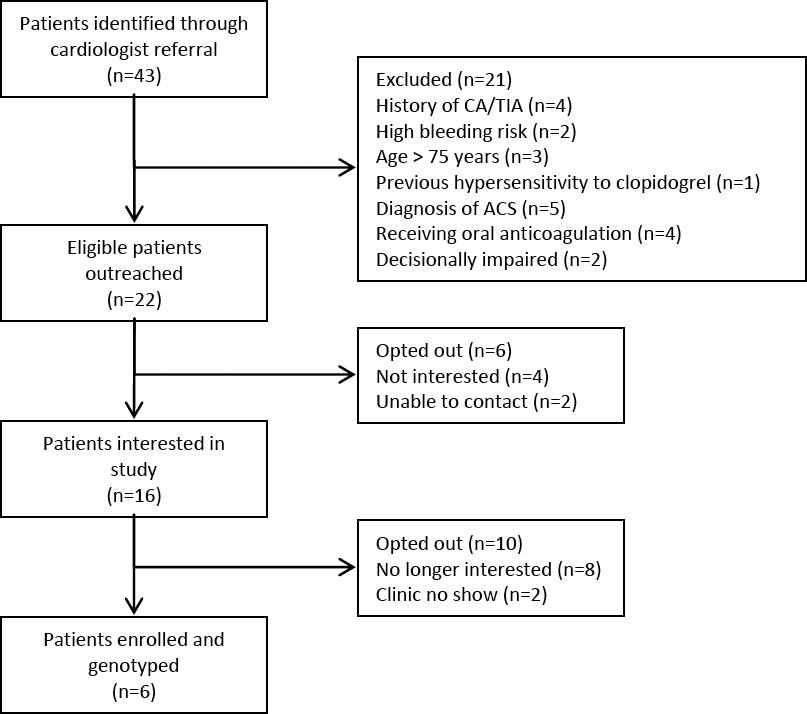
Figure 1 Study Enrollment Scheme.*CVA = cerebrovascular accident; TIA = transient ischemic attack; ACS = acute coronary syndrome.
Of the six patients genotyped, three possessed the wild-type CYP2C19*1/*1 genotype (Table 1). For these patients, the clinical pharmacist recommended use of clopidogrel 75 mg daily. The remaining three patients had variant CYP2C19 genotypes (i.e., *1/*2, *1/*17, or *2/*17). For the patient with the CYP2C19*1/*2 genotype (i.e., intermediate metabolizer), the clinical pharmacist recommended the use of ticagrelor 90 mg twice daily. For the patient who possessed the CYP2C19*2/*17 genotype, a combination of one loss-of-function allele (*2) and one increased function allele (*17), the clinical pharmacist recommended clopidogrel 75 mg daily. Although current Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines28 recommend using an alternative to clopidogrel in patients with the CYP2C19*2/*17 genotype, the 2011 CPIC guidelines available at the time the study was conducted did not discuss the CYP2C19*2/*17 genotype nor did the guidelines provide recommendations to utilize alternative therapy. For the patient who possessed CYP2C19*1/*17 genotype (i.e., rapid metabolizer), the clinical pharmacist recommended use of clopidogrel 75 mg daily with increased monitoring for bleeding.
DISCUSSION
This pharmacogenetics feasibility study identified that a relatively modest proportion of patients who required elective cardiac catheterization chose to be genotyped to guide their cardiologist’s choice of their antiplatelet therapy. The proportion we identified (27%) failed to meet our pre-specified feasibility criterion of greater than 50%. Nevertheless, of the patients who were genotyped, all (100%) had genotype results available prior to catheterization and all (100%) of the clinical pharmacist’s antiplatelet therapy recommendations were accepted by the cardiologists which met our pre-specified feasibility measure of greater than 80%.
Previous studies have evaluated CYP2C19 genotyping in non-acute care settings. For example, Ferreri and colleagues conducted a feasibility study within a community pharmacy in North Carolina and reported 43.9% patient participation rate and 100% acceptance rate of pharmacists’ recommendations for therapy modification in patients found to have variant genotypes.26 In this study, patients were recruited based upon prescriptions for clopidogrel, rather than by indication for clopidogrel. Swen and colleagues also conducted a feasibility study for CYP2C19 and CYP2D6 genotyping in a community pharmacy setting in the Netherlands.27 They found that a majority of patients consented to genotyping (58%); however, prescriber acceptance of recommendations was not assessed. Similar to the study by Ferreri and colleagues, this study recruited patients based upon prescription for clopidogrel without regard to indication. In both of these studies saliva samples were used for DNA analysis. Sweeny and colleagues suggested that CYP2C19 genotyping was feasible for patients with ACS in the cardiac catheterization laboratory as a majority (58%) of patients consented to genotyping.31 In that study, patients were recruited in the cardiac catheterization laboratory and blood samples were obtained for DNA analysis.
While our study focused on the specific application of CYP2C19 genotype-guided therapy for clopidogrel therapy, efforts to incorporate genetics into medication prescribing and management are becoming more common.32 The uptake in pharmacogenetics in clinical settings is being driven by the availability of evidence-based guidelines for valid and clinically actionable drug-gene pairs, as well as the incorporation of genetic information in EHR and development of associated clinical decision support tools.33 Clinical implementation of CYP2C19 genotyping is relevant to several other medications such as selective serotonin reuptake inhibitors, tricyclic antidepressants, proton pump inhibitors, and voriconazole.5,34,35 As such, our study represents one, among many, aimed at demonstrating the value of clinically-applied pharmacogenetics, a scenario that is increasingly plausible due to rapidly decreasing costs, increasing availability of genetic testing, and a commitment from federal agencies to support full implementation of EHR.36 In light of these developments, it is imperative for health systems to adapt available technology to optimize clinical care delivery and safeguard the privacy of patients. While clinical evidence demonstrating feasibility of ‘just-in-time’ genotyping for patients at risk for cardiovascular events in the ambulatory care setting is limited, there is significant opportunity to discover ways to apply pharmacogenetic testing to clinical practice and implement effective clinical pharmacy services to optimize patient outcomes.
While our study provides important insights into the feasibility of pharmacogenetic testing in the ambulatory care setting, it did have limitations. We aimed to enroll at least ten patients but were only able to enroll six patients. In addition to a smaller than projected number of referrals from the cardiologists, our study also saw a lower than anticipated patient acceptance rate (27%) which differed from previous reported studies. A potential explanation of this difference may be the recruitment strategy used as well as the inconvenience of the additional office visit for the purpose of consent/collection of saliva sample. Figure 1 illustrates this concern as 16 of the 22 patients who were originally interested, eventually lost interest or failed to show up. Conversely, in the Ferreri and colleagues study, initial recruitment efforts were performed at the point of dispensing in a face-to-face manner and Swen and colleagues performed house-visits for saliva sample collections. However, the six patients in our study did provide important information regarding the occurrence of genetic variants. We conducted this study in one integrated healthcare system. Other healthcare systems may identify different results. We only included patients who were to undergo non-emergent cardiac catheterization. Patients requiring emergent cardiac catheterization or pharmacogenetic testing for other indications may be more/less willing to consent to testing.
CONCLUSIONS
A relatively small proportion of patients undergoing non-emergent cardiac catheterization consented to pharmacogenetic testing; however, their cardiologists were receptive to clinical pharmacists conducting such testing and providing corresponding pharmacotherapy recommendations. The strategy for successfully implementing pharmacogenetic testing may be an important consideration, particularly as it relates to understanding patients’ concerns about such testing. Future studies should identify patient barriers to pharmacogenetic testing.















