Highlights
Momordica charantia L. is widely used in traditional medicine.
Momordica charantia L. act as anti-inflammatory.
Momordica charantia L. decrease of TNF-α
Introduction
Momordica charantia L. belongs to the Cucurbitaceae family and is known popularly as bitter gourd, bitter melon, kugua, or balsam pear1. The plant grows in tropical and subtropical regions, and especially the fruits and seeds are destined for therapeutic purposes2.
The several bioactive compounds of M. charantia have been recorded in the literature; they are classified as carbohydrates, proteins, lipids, triterpenoids, saponins, polypeptides, flavonoids, alkaloids, sterols and they have been used to treat various diseases and conditions since ancient times, such as cancer, asthma, bacterial, fungal and parasites infections, rheumatism, metabolic disorders and others, as can be seen in Table 1 1,3-5. As reported, M. charantia possesses various pharmacological activities already described, but there are also adverse effects associated to its use (hypoglycemic coma in children, abortion or even death in laboratory animals) that have been reported in the past years and which limit its wider application6.
Table 1. Main compounds already of Momordica charantia in literature.
The use of plants for therapeutic purposes is an old practice and is still very recurrent. Given the wide use and adverse effects related to M. charantia, it is of great importance to prove their safety. Very studies have analyzed the pharmacological action and adverse effects of M. charantia in different experimental models, however, they proposed high concentrations, so it becomes necessary to investigate if these effects happen even in lower concentrations and healthy conditions. However, they proposed high concentrations, so it becomes necessary to investigate if these effects happen even in lower concentrations and healthy conditions.
In this regard, the present study mainly aimed to evaluate the effects of Momordica charantia L. extract on human lymphocytes, especially on inflammatory, cytotoxic, genotoxic, and mutagenic aspects.
Methods
Chemicals
The reagents used in this study, sterile Histopaque® 1.077 g/mL, RPMI 1640 modified with 20 mM HEPES and L-glutamine, inactivated Fetal Bovine Serum (FBS), Phytohemagglutinin-M (PHA-M), Lipopolysaccharide (LPS), penicillin/streptomycin, gentamicin solution, quercetin, kaempferol, and rutin were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA). The Momordica charantia L. plant used in this study was obtained from a local market in March 2015 (Cunha Porã, SC, BRA). Analytical grade chemicals, such as methanol, gallic acid, acetic acid, chlorogenic acid, and caffeic acid were purchased from Merck (Darmstadt, HE, DEU).
Preparation of plant extract
The fruits and seeds of Momordica charantia L. were triturated and macerated in a hydroalcoholic solution at a concentration of 20 g per 100 mL of solvent at 25ºC for one week under daily shaking. This maceration process was repeated for three more weeks to exhaust the material. After this period, the crude extract was filtered and evaporated to dryness with a rotary evaporator to remove ethanol and water. Thus, we obtained the dry extract of Momordica charantia L. (MCE), used in the subsequent tests.
Chromatography
The high-performance liquid chromatography (HPLC-DAD) was performed using a Shimadzu Prominence Auto Sampler SIL-20A HPLC system (Shimadzu, Kioto, Japan) equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and the data were recorded with LC solution 1.22 SP1 software. Under gradient conditions, the reverse-phase was carried out using a C18 column, 4.6 mm x 150 mm, 5 µm. The mobile phase used was water containing 2% acetic acid (A) and methanol (B), and the gradient conditions consisting in 5% of B until 2min and changed to obtain 25%, 40%, 50%, 60%, 70%, and 100% B at 10, 20, 20, 40, 50 and 80min respectively, as described by Laghari7 with slight alterations. We analyzed the presence of six antioxidant compounds, namely, gallic acid, chlorogenic acid, caffeic acid, quercetin, rutin, and kaempferol, by comparing retention time and UV absorption spectrum with their respective standards. The flow rate was 0.7 mL/min, injection volume was 40 µL, and detection wavelength was 254 nm for gallic acid, 327 nm for caffeic and chlorogenic acid, and 365 nm for quercetin rutin and kaempferol. The samples and mobile phase were filtered through a 0.45 µL membrane filter (Millipore) and subsequently degassed by ultrasonic bath previously to use. Calibration curves were built using known standard compounds for quantitative measurement of different compounds in the extracts. All the analyses were carried out in triplicate and room temperature.
Ethical aspects
The Ethics Committee approved this Federal University of Pampa study under protocol number 27045614.0.0000.5323. All the volunteers signed the free and informed consent terms (TCLE).
Lymphocytes isolation
Initially, we collected peripheral blood from seven healthy self-declared volunteers following the Organization for Economic Cooperation and Development guidelines8. We used Histopaque-1077® (2:1) to separate the peripheral blood mononuclear cells (PBMC), which were transferred to a culture flask containing medium RPMI 1640 supplemented with 10% FBS, 1% penicillin/streptomycin (v/v), and 0.2% gentamicin (v/v) and maintained in an environment at 37ºC and 5% CO2 for 24h. After this period, we isolated the lymphocytes and stimulated them by adding PHA-M (1 mg/mL) for further experiments. Lymphocyte density was adjusted for each protocol and performed in triplicate.
Treatment schedule
The MCE was dissolved in RPMI 1640 with DMSO 3% (v/v) to final concentrations of 12.5, 25, and 50 µg/mL just before use. The concentrations selection was based on preliminary tests carried out in our laboratory (data not shown). Each concentration was tested, and the control groups included three replicates, analyzed after 24h of exposure to MCE. The negative control (NC) consisted of medium and DMSO 3%. The positive control (PC) contained medium and LPS (100 μg/mL) for the inflammatory parameters, and the other analyses, medium and 10 μM H2O2.
Lymphocytes viability
Lymphocytes were cultivated in sterile 24-well plates to a density of 1 x 105 cells/well and were exposed to previously described concentrations of MCE for 24h. Lymphocyte viability was assessed by the Trypan blue dye exclusion method based on cell membrane integrity; unviable cells do not have the intact membrane, thus uptake the dye and show blue staining9,10. Cell viability was expressed as the percentage of viable cells.
Micronucleus assay
Mutagenicity was evaluated using the micronucleus assay accomplished according to Schmid11. In this test, lymphocytes were seeded in sterile 24-well plates at a density of 1 x 105 cells/well and exposed to previously mentioned concentrations of MCE for 24h. Subsequently, an aliquot of each culture was collected to prepare the slides, which were fixed with PA methanol, stained with methyl acid orange, Giemsa-basic (Laborclin), and dried at room temperature. Then, using an optical microscope (Olympus CX21 Led) with 1000x magnification, 500 cells per slide were analyzed and scored according to the presence or absence of micronuclei. The results were expressed as a percentage of cells with micronuclei.
Comet assay
Genotoxicity was assessed utilizing the comet assay and realized according to Singh et al12 and Tice et al13. Lymphocytes were cultivated in sterile 24-well plates at a density of 5 x 104 cells/well and exposed to earlier selected concentrations of MCE for 24h. Afterward, an aliquot of each culture was homogenized with low melting point agarose 0.75% (w/v) and distributed on slide pregelatinized with normal melting point agarose and then covered with a coverslip up until solidification. The slides were immersed into a cold lysis solution (NaCl 2,5 M, EDTA 100 mM, Tris 10 mM, pH 10.0 and Triton X-100 1% with 10% of DMSO) for 24h. In sequence, electrophoresis was carried out (25 V; 300 mA) in 300 mM NaOH/ 1 mM EDTA buffer, pH > 13 for 20 min, inducing an alkaline denaturation. Then, the slides were subjected to the neutralizing solution, fixed, and stained with silver nitrate solution. The nucleoids were evaluated under an optical microscope at 400 × magnification and ranked in scores of 0 (no migration of DNA) to 4 (maximum migration of DNA) according to the damage levels; 100 nucleoids were counted in all slides. The DNA damage index can vary from 0 (all nucleoids with no migration) to 400 (all nucleoids with maximum migration of DNA).
Inflammatory parameters
The lymphocytes (5 x 104 cells/well) were seeded in sterile 24-well plates and exposed to previously described concentrations of MCE. Except for the negative control, all the cultures also received LPS (100 μg/ mL) and were incubated for 24h. After this period, the inflammatory parameters, interleukin-6 (IL-6), interleukin-10 (IL-10), cyclooxygenase-2 (COX-2) activity, Tumoral Necrosis Factor-α (TNF-α), and Nitric Oxide (NO) were measured using ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Statistical analysis
The data were expressed as mean ± standard deviation and performed all analyses using specific statistical software. The comparisons between groups were realized using one-way analysis of variance (ANOVA), followed by Bonferroni’s posthoc test. The results were considered statistically significant for p<0.05.
Results
Chromatography
The quantification of previously selected antioxidant compounds showed that quercetin (21.75 µg/mL), gallic acid (15.11 µg/mL), kaempferol (13.46 µg/mL), and chlorogenic acid (12.26 µg/mL) were the most constituents present in MCE (Table 2).
Table 2. Quantification of active principles in MCE.
| Active principle | Concentration in the sample (µg/mL) | Dry weight of the sample (mg/mL) | Active principle concentration in the extract (µg/g of the plant) |
|---|---|---|---|
| Quercetin | 21.75 | 22.3 | 975.22 |
| Gallic Acid | 15.11 | 677.73 | |
| Kaempferol | 13.46 | 603.77 | |
| Chlorogenic Acid | 12.26 | 549.79 | |
| Caffeic Acid | 8.75 | 392.26 | |
| Rutine | 8.34 | 373.94 |
Lymphocytes viability
None of the concentrations of MCE investigated in our study affected lymphocytes viability (Figure 1a), which was about 98% in all concentrations of MCE evaluated.

Figure 1. Lymphocyte’s viability (1a), micronucleus frequency (1b) and DNA damage index (1c) in human lymphocytes exposed to different concentrations of MCE. Data are expressed as mean ± standard deviation, n=3, performed in triplicates and analyzed by ANOVA followed by Bonferroni’s post hoc. We considered significant results with p<0.05. Different letters mean statistically different values. NC = Negative Control; PC = Positive control (H2O2 10 µM).
Micronucleus assay
The results demonstrated that MCE did not increase micronucleus frequency in lymphocytes compared to the negative control in the tested concentrations (Figure 1b).
Comet assay
The comet assay data showed that MCE induced DNA damage concentration-dependent than the negative control group (Figure 1c).
Inflammatory parameters
Our results showed that the tested concentrations of MCE did not affect the production of IL-6 (Figure 2a), IL-10 (Figure 2b), COX-2 activity (Figure 2c), and production of NO (Figure 2d). On the other hand, MCE caused a reduction of TNF-α (Figure 2e) under the experimental conditions in the concentrations of 25 µg/ml and 50 µg/mL concerning the negative control group.
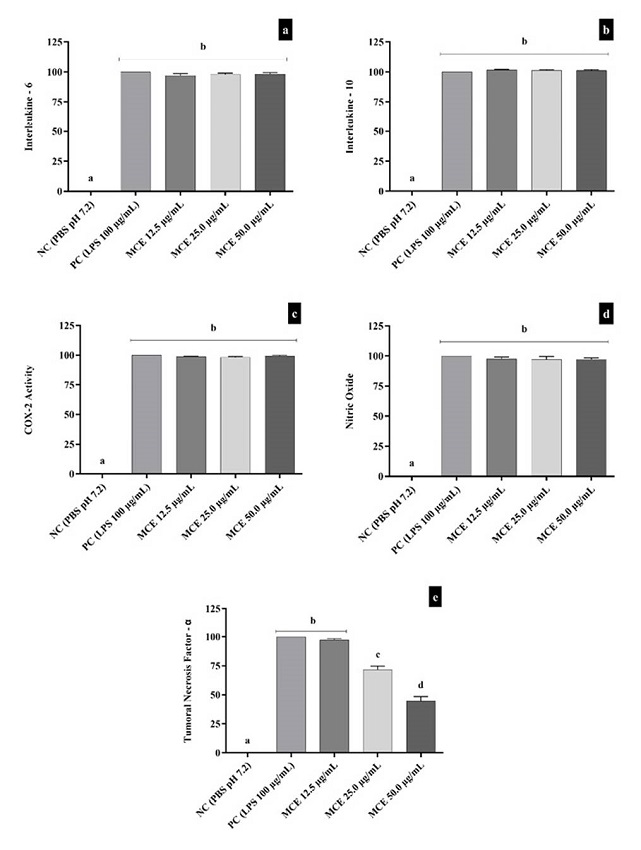
Figure 2. Effect of MCE on IL-6 (2a), IL-10 (2b), COX-2 activity (2c), Nitric Oxide (2d) and TNF-α (2e). Data are expressed as mean ± standard deviation, n=3, performed in triplicates and analyzed by ANOVA followed by Bonferroni’s post hoc. We considered significant results with p<0.05. Different letters mean statistically different values. NC = Negative Control; PC = Positive Control (LPS 100 µg/mL).
Discussion
Bitter melon is a climber classified as monoecious, belonging to the Cucurbitaceae family, commonly used for food consumption14. Due to the biological properties attributed to the plant, M. charantia also receives several uses in traditional medicine, and studies have investigated a variety of activities, which are related to phytochemical composition14,15. In our study, we identified the presence of six antioxidant constituents in the plant extract, gallic acid, chlorogenic acid, caffeic acid, quercetin, rutin, and kaempferol, most compounds. The gallic and caffeic acid16 and chlorogenic acid17 have already been described in the M. charantia composition, varying only in concentrations. The amount of quercetin found in the MCE sample also was reported in other studies. The work of Pereira18 related that quercetin was the main flavonoid found and Supe et al19 demonstrated that the concentration of quercetin was between 0.05 to 0.2% in the analyzed sample. Sathasivam et al20 also found kaempferol in high amounts in the fruit extract of MCE, corroborating our findings.
In our study, MCE did not affect lymphocytes viability which corroborates with other authors21, who found that MCE was not cytotoxic against primary epithelial cells even after 5 days of exposure.
Agrawal et al22 demonstrated that a single administration of MCE at doses of 500, 1000, and 1500 mg/kg of body weight 24h before the administration of cyclophosphamide expressively prevented the formation of micronucleus in mouse bone marrow cells, which agrees with our findings that showed MCE did not affect the frequency of micronucleus under the tested conditions. Other studies have shown that aqueous extract of M. charantia and methanolic extract of the plant’s leaves have anti-mutagenic activities23,24.
Our findings show that MCE induced DNA damage in a concentration-dependent manner; this damage is identified by a kind of tail like a comet formed by the DNA fragments.
Lii et al25 found that MCE induced DNA damage in four human cancer cell lines, Hone-1 nasopharyngeal carcinoma cells, AGS gastric adenocarcinoma cells, HCT-116 colorectal carcinoma cells, and adenocarcinoma cells of CL1-0 lung at concentrations between 0.25 to 0.35 mg/mL in exposition up to 24h. Nevertheless, Ganguly26 reported a reduction of DNA damage in circulating lymphocytes of normal mice and in a mouse skin papilloma induced by 7,12-Dimethylbenz[a]anthracene (DMBA) administration following the oral administration of 100 µL and 50 µL of M. charantia aqueous extract daily for 3 months. Corroborating with our results, Li27 data suggests a potential antitumoral activity of MCE. However, Ganguly26 results showed a decrease in DNA damage, and this discrepancy can be explained by the different experimental models and time of exposure to M. charantia.
Our results of inflammatory parameters showed that IL-6 production was not affected by MCE. IL-6 is a pro-inflammatory cytokine readily produced in cases of infections and tissue injuries28. Cao29 demonstrated that cucurbitane-type triterpenoids compounds isolated from the fruit of M. charantia with IC50 values of 0.028 to 1.962 µM could inhibit the production of IL-6 in LPS-stimulated bone marrow-derived dendritic cells, and the authors attributed these effects to interactions of hydrogen bonds between the protein residues and hydroxyl groups and sugar rings of triterpenoids compounds, which were visualized by molecular docking. Another study showed that the expression of IL-6 in adipose tissues and brown adipose tissues of diet-induced obese mice was lower in animals fed with the fruit of M. charantia in the concentrations of 2% and 5%30, the authors associated these results to the improvement of insulin resistance and fat deposition in the rodent model. It is noteworthy that IL-6 is associated with adipose tissue inflammation related to insulin resistance. IL-10 is a regulatory cytokine that possesses anti-inflammatory properties31. Fachinan31 showed the protective anti-inflammatory effects of M. charantia in a study with diabetic rats treated with M. charantia fruit juice (10 mL/kg body weight) for 28 consecutive days. In addition, Ünal32 also reported an increase of IL-10 in rats with colitis induced experimentally with 300 mg (kg/day) of MCE for 6 weeks orally. Our results showed that MCE did not affect the production of IL-10 in isolated human lymphocytes; this discrepancy could probably be due to the experimental model, different times of exposure, and concentrations of MCE.
In our study, there was no alteration in COX-2 activity; already Chao33 observed a decrease of COX-2 enzyme expression levels in rats with induced sepsis and treated for 4 weeks with 4 g daily of a test feed containing 10% of MCE lyophilized powder. Ali34 also observed a reduction in an animal model of hepatocarcinogenesis in which the rats were treated with M. charantia methanol extract (40 mg/kg body weight) for 30 days. Additionally, Yang, Yang (35) identified inhibition of COX-2 mRNA expression in RAW 264.7 with inflammatory process induced by LPS (1 µg/mL) for 24h and pre-incubated with M. charantia methanol extract (100 and 200 μg/mL) for 30min. Differences in the cell lines can explain these divergences, treatment protocols applied, and since cells with high inflammatory status have a more significant response to the compounds with potential anti-inflammatory effects present in the MCE than regular cell lines.
We observed that MCE did not induce alterations in the production of NO, opposite to Li35, which described inhibition of NO production in mouse macrophage-like cell line RAW 264.7 with inflammation induced by LPS and exposed to different M. charantia fruit extracts for 24h, being that 50 µg/mL ethanolic extract which presented the most NO levels decrease. This discrepancy can be related to the cell line, time of exposure, and the LPS concentration selected for inflammation induction (1 µg/mL) lower than the concentration applied in our study. Dwijayanti36 also reported a reduction of NO in a dose-dependent manner in rats hepatocytes treated with interleukin-1β (IL-1β) and M. charantia methanol extracts (50 µg/mL, 100 µg/mL and 200 µg/mL) for 8h. These variations may be associated with cell lines, exposure time to MCE, and especially to the IL-1β inflammation induction therapy37.
In our study, we also observed that MCE was able to reduce TNF-α levels, which is in agreement with results found by Lee38, who demonstrated that MCE could dose-dependently reduce the production of TNF-α in RAW 264.7 murine macrophages treated with a concentration of 12.5% v/v (5 mg/ mL of soluble MCE) for 4h. Cheng39 also observed this reduction in FL83B cells with induced inflammation by TNF-α and treated for 24h with a mixture containing the triterpene isolated from M. charantia.
In conclusion, we demonstrated in our study that quercetin, gallic acid, kaempferol, and chlorogenic acid were the primary compounds found in MCE analyzed. The MCE did not induce cytotoxicity, alteration of micronucleus frequency, or interference in the production of IL-6, production of IL-10, COX-2 activity, and NO production, but caused DNA damage and a decrease of TNF-α in purified human lymphocytes exposed to MCE for 24h. For that reason, we suggest that MCE modulates inflammatory response in human lymphocytes via suppression of TNF-α. The applications of MCE were related to its use as a possible nutraceutical in the future, evaluating the safety of its use. In the literature, adverse effects associated with its use have been reported in recent years limiting its applications6, however, these studies used high concentrations of MCE. Therefore, we proposed exposure to lower concentrations and healthy conditions, with the intention of observing whether these effects persist even in these circumstances. Although studies have reported biological activities of MCE and among them the anti-inflammatory, our research proposed a mechanism through which the MCE could modulate the inflammatory response in human lymphocytes.


























