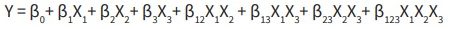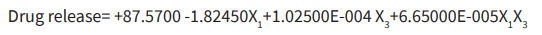Highlights
Nanoparticulate oral drug delivery system can be substitute to conventional dosage formEthocelTM as a polymer and Kolliphor® P 188 can be applied to develop stable nanoparticulate formulation. Statistical technique like factorial design would be helpful in formulation optimization.
Introduction
From last two decades, application of nanoparticulate drug delivery system hiked in various areas such as pharmaceutical field, paint industry, electronics, construction, packaging industry, food technology, energy, health care systems, automotive, and defense system. As nanoparticles (NPs) posses' particle size in between 1 and 100 nm has colloidal nature which enhanced their applicability in pharmaceutical field for encapsulation, absorption and dispersion of active pharmaceutical ingredient (API) in various dosage forms. NPs based Drug delivery system (DDS) can be developed with variation in their physicochemical properties by applying wide diverse materials such as lipids, polymers and inorganic constituents which helps to enhance their applications also1. Most polymer based nanoparticles (PNPs) developed by precipitation, emulsification and salting-out methodologies have high solubility and permeability enabling them to be stable with slow drug release over a long period of time making these PNPs efficient nanocarriers for various anti-cancer agents2-3. Iron is an important element which plays a crucial function in the metabolism of human body4. In various diseased conditions such as transfusion hemosiderosis, genetic diseases, or hereditary haemochromatosis iron get absorbed by the body in excess than normal which leads to develop iron overload. This excess absorption of iron leads to retention in vital organs like heart, liver, pancreas which leads to failure after long time if not treated properly. Currently, the treatment for iron overload involves proper diet modification alongwith therapeutic agents such as Deferasirox. Thus the patients suffering from iron overload is usually suggested to administer with Deferasirox having half life between 8-12 hrs. It is also found that tumor cell growth depends on iron whereas application of chelators reduces the iron uptake which can be one of the treatments to develop conventional cancer therapies5. Deferasirox is the first oral medication approved by FDA in 2005, and European Medicines Agency (EMA) in 2006 for the treatment of chronic iron overload disorders. It is iron chelator used to treat hemosiderosis, iron overload and thalassemia. It also detected with anticancer activity against various cancer cell lines which reflects requirement of large amounts of iron to cancer cells as compared to normal cells for mediation of their quick DNA replication and proliferation. To determine effective therapeutic effects, re-formulation of DFX-loaded NPs with high solubility and bioavailability were developed. DFX has a short plasma half-life and can be deliver systemically using long courses of intravenous (IV) infusion or intramuscular (IM) injection which can be challenging also. These rotes of administrations found to be difficult to attain therapeutic concentrations of drug which may require higher doses and may cause systemic toxicity. Thus use of mucoadhesive system prevent enzymatic degradation and increases the contact time with the mucosa to attain therapeutic concentration6.
Therefore, it would be beneficial to develop Deferasirox nanoparticles (DNPs) to lessen number of doses and toxicity which will facilitate to improve the therapeutic efficacy of Deferasirox and helps to reduce cancer cell growth in cancer treatment. In current research, DNPs were assessed for particle size detection, entrapment efficiency, drug release, and cell viability analysis and stability studies.
Materials and Methods
Materials
Novartis Pvt. Ltd., Mumbai, India gifted Deferasirox whereas Ethyl cellulose 7 premium (percentage of ethoxy content: 48 and viscosity 6-8cps) as EthocelTM and Poloxamer 188 as Kolliphor® P 188 was gained as gift samples from BASF, Mumbai, India. AR grade reagents were applied throughout study.
Methods
Preparation of Nanoparticles by Emulsion Solvent Evaporation Technique:
NPs were developed using conventional solvent-displacement method. An initial screening of the main parameters that commonly have impact on NPs formation was executed as initial step of the study. The studied parameters were stirring rate, time of stirring, type and stabilizer concentration. An adequate amount of Deferasirox and EthocelTM were liquefied in ethyl acetate of 10 mL as organic phase at 370C with magnetic stirrer. This solution was injected into 40 mL of a solution of the surfactant during 4 min under high-speed stirring at 370C (Ultraturrax® T25, IKA, Germany). Finally, the residual ethyl acetate was eliminated by continuous stirring for 24 hrs at 25°C. The remaining surfactant and un-encapsulated drug was then removed by ultracentrifugation at speed of 11,000 rpm for 40 min. To evaluate the outcome of process conditions, NPs were prepared at different stirring rates: 20,000, 22,000, and 24,000 rpm, with a high-speed stirrer (Ultraturrax®, T25, IKA, Germany). Drying of batches was performed in incubator at 50°C7.
Factorial design experiments
Factorial design of 23 schemes was applied for existing research work including 3 factors at 2 levels and assessed for interactions among chosen variables'. Concentration of polymer EthocelTM (X1), surfactant concentration Kolliphor® P 188 (X2) and rpm (X3) at two different levels were selected as variables Table 1.
Table 1: Experimental variables of factorial design with their coded levels and actual values of EthocelTM (mg), concentration of Kolliphor® P 188 (%) and RPM, Results of % Drug release, % Drug Entrapment Efficiency
| Batch | Factor 1 EthocelTM (mg) | Factor 2 Kolliphor® P 188 (%) | Factor 3 RPM | Response 1 % Drug Release | Response 2 % Drug Entrapment Efficiency |
|---|---|---|---|---|---|
| F1 | 10 | 0.5 | 20000 | 84.82±0.49 | 70.19±0.31 |
| F2 | 20 | 0.5 | 20000 | 77.53±0.16 | 81.71± 0.37 |
| F3 | 10 | 1.00 | 20000 | 84.65±0.42 | 67.34±0.64 |
| F4 | 20 | 1.00 | 20000 | 78.73±0.42 | 88.06±0.56 |
| F5 | 10 | 0.5 | 22000 | 87.34±0.11 | 65.54±0.44 |
| F6 | 20 | 0.5 | 22000 | 84.2±0.03 | 77.55±0.80 |
| F7 | 10 | 1.00 | 22000 | 85.42±0.61 | 69.46±0.51 |
| F8 | 20 | 1.00 | 22000 | 81.41±0.71 | 74.94±0.15 |
The study includes matrix design with responses such as percentage of drug entrapment efficiency (DEE), percentage of drug release mentioned into Table 1. Formulation was developed and was assessed as per statistical design using Design-Expert Version 7.0.0 software (Stat-Ease Inc., Minneapolis, MN 55413, USA). Responses investigated as per effects of independent factors mathematically presented in following quadratic equation:
Where, β0 is arithmetic mean response of formulation runs; β1, β 2 and β 3 are estimated coefficients for selected (X1, X2, X3) independent factors; β12, β13, β23 and β123 are estimated coefficients for (X1X2, X1X3, X2X3 andX1X2X3 resp.) interaction and Y is the dependent variable.
Selected factors varied for low to high level signify average outcome due to main effect terms (X1, X2 and X3). X1X2X3 are interaction terms demonstrate variation in responses on modifications of three selected factors. Degree of coefficient and mathematical outcome was considered for interpretation of effects of variables8. The optimization technique found to be more effective and economic due to its minimum experimentation with less time as compared to conventional techniques for developing dosage forms9.
Acceptance of appropriate model was detected by Analysis of variance (ANOVA). Response surface curves interpreted for representation of interaction effects and main effects. Developed formulations were evaluated thrice for all experiments and outcomes were quoted as mean ± SD where significance was detected for p<0.05.
Particle Size Analysis:
Malvern Zeta sizer was applied for detection of poly- dispersity index (PDI) and average particle size (z average) of developed NPs formulation using laser dynamic light scattering technique. NPs suspension was diluted to 1/50 v/v in water for detection of particle size where n=3. Particle size distribution of NPs sample specifies PDI value. The presence of aggregate detected with elevated value of PDI and various particle size ranges. Thus, lower stability and homogeneity could be the outcome of elevated value of PDI10.
Zeta Potential:
Malvern Zeta sizer was applied for detection of zeta potential by diluting 50 times the developed NPs formulation 50 times with distilled water. The particle surface charge detects stability of NPs which was designated by zeta potential11.
Infrared Analysis:
IR spectra of Deferasirox, EthocelTM, Deferasirox and EthocelTM physical mixture (maintained at 40 ± 2°C/75 % ± 5% RH for 2 month) were scanned for absorbance and recorded by IRAffinity-1, Shimadzu12.
Differential Scanning Calorimetry (DSC):
DSC thermo gram of Deferasirox, EthocelTM, optimized formulation and its physical mixtures (maintained at 75 % ± 5 % RH and 40 ± 2°C for 60 days) verified by means of Shimadzu DSC-60 calorimeter and examined 10°C per minute as heating rate with 10 - 2000C temperature range13.
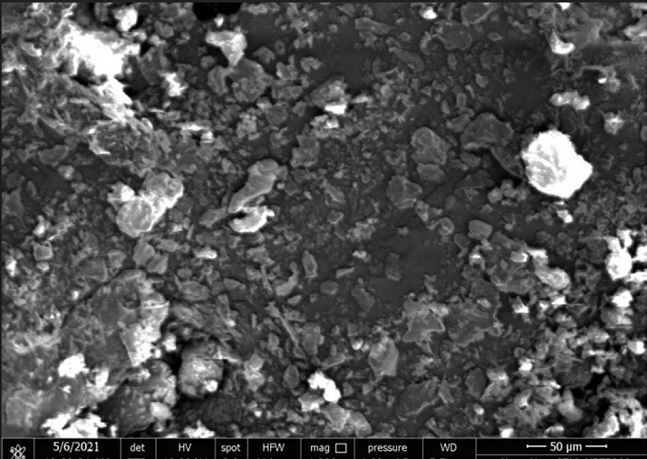
Scanning electron microscopy (SEM) study:
Surface morphology and shape of DNPs was evaluated using SEM. Metal stubs containing developed formulation with conductive gold coating along-with sputter coater loaded to instrument for capturing images14.
X-ray diffraction:
Deferasirox, polymer and DNPs were studied for crystallinity using XRD and recorded graph. Scanning was done up to 2 ranges between 2 and 90 µ15.
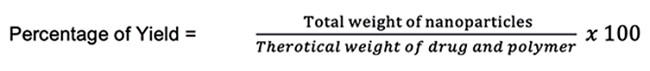
Percentage of yield
Developed formulations were evaluated for percentage of yield by weighing the NPs on collection and calculated as follows.
Drug entrapment efficiency:
Developed DNPs (equivalent to 6 mg of Deferasirox) were solubilsed at 100 rpm in 100 mL of ethyl acetate at RT and subjected at 10000 rpm for centrifugation for half an hour, followed by UV Spectrophotometric evaluation of supernatant at 315 nm (Jasco, Japan)16-17.
In-vitro Drug Diffusion:
Simple diffusion cell apparatus was applied for detection of drug release from developed NPs using in-vitro method which was unwrapped at both ends. One end fixed with eggshell membrane which provided as a donor compartment. Recently prepared phosphate buffer saline having pH of 6.5 was applied as diffusion medium. Eggshell membrane was saturated near about 12 hrs in the diffusion medium. The medium was agitated by means of the magnetic stirrer followed by maintaining at 37±0.5 0C. 5 mL of sample was removed as per schedule and subjected to spectrophotometer at 245 nm18.
Mucoadhesive study:
Potentiality of each developed batch for mucoadhesion was detected by adapting the method. Mucosal tissues of sheep oral cavity gained from local abattoir were cut into 1cm2 area followed by mounting on glass slide. About 100 NPs were spread onto the wet rinsed oral tissue specimen. Glass slide hang to one of the groves of USP tablet disintegration test apparatus containing 400 mL phosphate buffer (pH 6.5) maintained at RT. Mounted tissue kept in upward and downward motion at 20 rpm. Immediately, the time taken for entire cleansing of NPs from the tissue surface was noted19.
Cell Viability Studies:
Cell culture materials and maintenance:
National Centre for Cell Sciences, Pune, India offered human breast cancer cell line Michigan Cancer Foundation-7 (MCF-7). Minimal essential medium (MEM), trypsin phosphate versene glucose (TPVG), antibiotic antimycotic solution 100X and fetal bovine serum (FBS) were obtained from HiMedia laboratories. MEM enriched with FBS (10 %) and antibiotic antimycotic solution (1 %) was applied for maintenance of cells. Growth of cells was detected at 37 °C and 5 % of CO2 atmosphere in tissue culture flasks.
Anti-tumoral activity using human cancer cell line
DNPs formulation, pure drug Deferasirox was screened using human breast cancer cells (MCF-7) for antitumor activity. The anti-tumoral activity is estimated using cell viability assay. The cell viability was evaluated using colorimetric based MTT assay which is as per principle that colorless tetrazolium salt MTT ([3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide]) is reduced by viable cells to purple formazan crystals. The absorbance recorded at 570 nm is direct related to cell viability.
96-well plate was applied for seeding of MCF7 cells (2 x105 cells/mL) and subjected for incubation at RT in atmosphere containing 5 percentage of CO2 for 48 h. After 48 h, medium was discarded. DNPs formulation (1 µg/mL, 5 µg/mL , 10 µg/mL , 50 µg/mL and 100 µg/mL) was applied to cells in MEM medium(supplemented with 1 percentage of fetal bovine serum, 1 percentage of antibiotic antimycotic solution) and cells were subjected for incubation for one day. DNPs formulation was prepared in 2 % of DMSO solution (prepared in phosphate buffer saline) so that final concentration of DMSO for each treatment will be 0.2 % of DMSO. Along with DNPs formulation, cells were also treated with 0.2 % of DMSO solution alone (prepared in phosphate buffer saline) which was applied as a control. After 24 h, medium was discarded followed by phosphate buffer saline wash to the cells. All wells were charged with MTT solution ( 40 µg/well) and plate was subjected for incubation at RT in 5 % of CO2 atmosphere for 4 h. After 4 h, carefully supernatant was disposed of and DMSO (200 µL) was supplied to all well to liquefy formed formazan crystals. Optical density was measured using a microtitre plate reader (Synergy HT, BioTek Instruments Inc., USA) at 570 nm. The same procedure was followed for pure drug Deferasirox (1 µg/mL, 5 µg/mL, 10 µg/mL, 50 µg/mL and 100 µg/mL), surfactant polaxomer (1 µg/mL, 5 µg/mL, 10 µg/mL, 50 µg/mL and 100 µg/mL) for evaluating its anti-tumoral activity.
The cell viability was calculated using following formula,
Where OD is optical density
Data are represented as mean ± SD (standard deviation). Concentration of nanoparticles showing ≥ 90 percentage of cell viability was considered safe20-21.
Stability Studies: Developed NPs were set aside in hermetically sealed glass containers and subjected at various temperatures such as 4°C, RT and 45 °C. API content was detected in frequency of Ist, IInd, IIIrd and IVth week (Harmonized Tripartite Guidelines 2003).
Results and Discussion
Statistical analysis
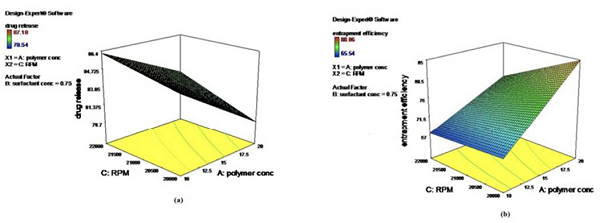
Surface responses of DoE are provided (Table 2 and Figure 1). Analysis of statistics governs number of experiments, precision and its impact on outcome of experiment that estimate response variables, conduction of trial batches to decide mainly potential model, application of obtained data to mathematical tools and generation of optimum responses by applying independent formulation variables obtained values. Factorial design, well accepted statistical experimental tool employed for current study to develop formulation by considering interactions among chosen factors. To estimate influence independent variables on properties and execution of formulation, 23 full factorial design was employed. Polymer proportion, surfactant concentration and rpm as independent variables at two levels were assessed its influence on percentage of drug release, percentage of DEE. The responses obtained are specified in Table 1. As per outcome from trial formulations, investigated responses were fixed in the 23 factorial design to get model equations for responses evaluated in this investigation. All models were statistically estimated by application of one-way ANOVA (p <0.05). Outcome of experiments studied were greatly influenced by selected variables detected from achievement of regression analysis and ANOVA. Percentage of drug release of DNPs (Table 1) was found in between 77.53±0.16 % to 87.34±0.11 %. As per regression analysis, significant release of API from DNPs was influenced variables applied (r2=0.8454). Main influence of polymer concentration and rpm elucidated with comparative magnitudes of regression coefficient. Response surface plot depicted drug release increases with decrease in polymer concentration and rpm (Figure 1). High percentage of polymer concentration increases drug entrapment of drug. Regression equation for percentage of drug release as follows:
(r2=0.8454 F value =7.29, p<0.05, i.e. significant) As per Table 1, variables significance was detected (r2=0.9161) on DEE and varied from 65.54±0.44 to 88.06±0.56. Thus, escalation in polymer concentration and rpm increases DEE. Regression equation for DEE as follows:
(r2= 0:9937, F value =14.55, p<0:05, ie. significant). Optimized DNPs noticed drug release after 6 h of 82.62%±1.04, DEE of, 72.64%±0.2 with small percentage of error valuesof 1.04 and 0.20, respectively (Table 1). Obtained model equations' validity and application of optimization model with its description helped to evaluate percentage of error. Low percentage of error was detected which designated that full 23 factorial design was suitable as per achieved mathematical models.
3D response surface plot represented augmentation in percentage of drug release with decrease in rpm and polymer concentration (Figure 1) whereas polymer concentration escalation hikes DEE. (Figure 1).
Particle size analysis
The particle size as well as PDI of formulation was evaluated. The nature of drug delivery from the formulation depends on size of NPs. Mean particle size of NPs was established to be 428.3 nm and PDI 0.626. Thus developed NPs are suitable for administration as per obtained particle size and PDI.
Zeta potential determination
Zeta potential of Deferasirox loaded EthocelTM nanoparticles for optimized batch was determined and it was found -14.4 mV. Zeta potential of Deferasirox loaded EthocelTM nanoparticles for optimized batch was detected with -14.4 mV which indicates moderate stability without agglomeration. Free carboxylic acid groups of EthocelTM polymer leads to development of negative surface charge. The possible effects of surface charge may affect the in-vivo life span of the natural drug delivery system.
Fourier Transform Infrared (FTIR) Analysis:
IR spectrum of Deferasirox, EthocelTM and Deferasirox + EthocelTM physical mixture were studied. IR of Deferasirox showed peaks at 3194.12, 3086, 2954 cm-1(N-H Stretching), 1666.50 cm-1 (C=O stretching), 1612 cm-1(aromatic, C=C stretching). IR spectra with characteristic peaks for physical mixture of Deferasirox + EthocelTM with neither deletion of existing peak nor addition of new peak in the spectrum indicated compatibility excipients with Deferasirox. The current study outcome revealed development of stable formulations.
Scanning electron microscopy (SEM) study:
Morphology of NPs was studied by SEM and shown in Figure 2 SEM of the optimized batch showed the smooth surface with spherical shape, may be due to optimum polymer and Kolliphor® P 188 concentration (Figure 2).
Differential Scanning Colorimetry:
Deferasirox, EthocelTM and DNPs were studied for characterization of structure, physical state and crystal characterization. Sharp endothermic peak was detected as per DSC thermo gram at 260.95°C due to liquefaction of API. Thermal transition at 227.66°C is detected with melting of EthocelTM. DSC of DNPs formulation showed thermo gram without API peak. DSC studies detected that Deferasirox was homogeneously dispersed in nanoparticles.
X-ray diffraction study
XRD spectra for Deferasirox, EthocelTM and DNPs were studied. XRD with distinctive deep peaks was detected by Deferasirox between 20 of 10 and 14 and 17. Absence of crystalline peaks of DFX in developed NPs indicates drug dispersion in polymer at molecular level with development of its amorphous nature of formulation.
Percentage of yield:
Developed formulations were detected for percentage of yield by considering weight of NPs on drying in between 30 % and 50 %. As per the obtained percentage of yield of developed formulations, enhancement of polymer proportion slightly increased yield.
Entrapment efficiency:
The entrapment efficiency was compasses between 65.54±0.44 to 88.06±0.56. The DEE of optimized formulation was established to be 72.64±0.24%.
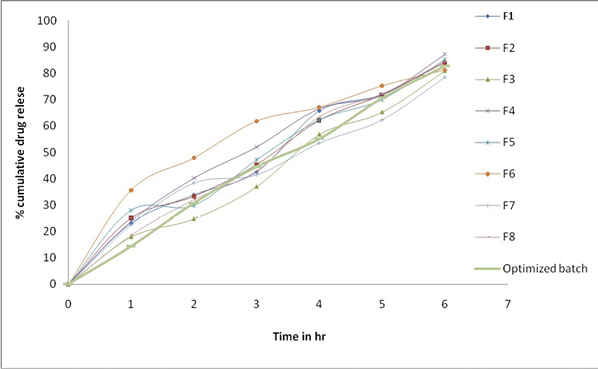
Drug release study:
Developed DNPs were studied for in-vitro drug release and presented as Figure 3. Sustained release was detected from DNPs formulated using EthocelTM up to 6h as shown in Figure 3. The Drug release of optimized formulation was established to be 82.62±1.04. Drug release mechanism determined by subjecting release data to various models like Korsemeyer, Higuchi, zero order and Peppas. All the formulation followed Higuchi Plot equation which indicated that NPs followed more than one type of release phenomenon and it appeared to be by Non-Fickian mechanism. (Table 2).
Mucoadhesion Test:
Mucoadhesion for various formulations of NPs were studied. Adherence capacity of developed NPs to mucosa at site of absorption for long duration was evaluated by mucoadhesion study. The escalation in polymer concentration increases mucoadhesion. Percentage of mucoadhesion was obtained in between 72 ±0.361 % to 89±0.489 %.
Anti-tumoral activity study:
Anti-tumoral activity of Deferasirox NPs, Deferasirox (pure drug) and corresponding Kolliphor® P 188 (surfactant) was studied using cancer cell line of human breast (MCF-7) by MTT assay. All the three pure drug, surfactant and nanoparticle formulation were studied using cancer cell line of human breast (MCF-7) by MTT assay in range of concentration from1 μg/mL to 100 μg/mL Initially API and DNPs were studied at higher dose (100 μg/mL to 500 μg/mL) and were detected to be cytotoxic at all the doses tested. Therefore, cell viability assay was repeated using lower concentrations.
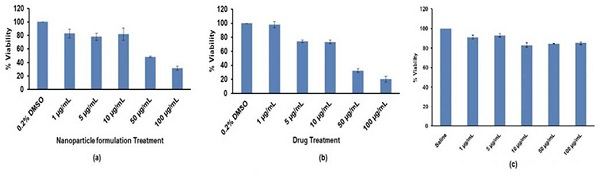
1) Cytotoxicity of NPs formulation
Cytotoxicity of NPs formulation was examined and presented in Figure 4 (a). It was examined that at higher dose concentration from 50 µg/mL up to 500 µg/mL less than 50% cells were viable. At lower Deferasirox nanoparticle formulation dose (1 µg/mL, 5 µg/mL and 10 µg/mL) ~80% cells were viable (Figure 4 (a)).
2) Cytotoxicity of drug
Cytotoxicity of drug Deferasirox treated cells are presented in Figure 4 (b). Pure drug Deferasirox treated cells also showed dose dependent cell viability. It was examined that as pure drug dose increases from 50 µg/mL up to 500 µg/mL there is decrease in cell viability. Pure drug was not toxic to breast cancer cells at lower concentration (1 µg/mL, 5 µg/mL and 10 µg/mL) (Figure 4 (b)).
3) Cytotoxicity of Kolliphor® P 188
Cytotoxicity of Kolliphor® P 188 was studied and presented in Figure 4 (c). Kolliphor® P 188 is safe to use as a surfactant and can be added in formulation which is detected as per obtained results. It showed non-toxic effect on MCF7 cells at all the tested concentrations (1 µg/mL to 100 µg/mL). Results of cell viability assay concluded that pure drug Deferasirox and Deferasirox nanoparticle formulation showed anti-tumoral activity at tested concentration from 50 µg/mL up to 500 µg/mL. Kolliphor® P 188 showed higher cell viability indicating suitability of Kolliphor® P 188 as surfactant (Figure 4(c)).
Stability Studies:
Short term accelerated stability study was executed at 40±2 ºC and 75±5 % RH for 3 months and the outcomes are mentioned in Table 3. After conducting short term accelerated stability study at 40±2 ºC and 75 ±5 % RH for 3 months, the optimized nanoparticle formulation was tested for DEE and drug release. After performing evaluation of optimized nanoparticle formulation, % drug release and DEE of formulation was established to be 70.68±0.002 and 79.57±0.54 respectively indicating developed NPs formulation was stable for the storage period. (Table 3) (Harmonized Tripartite Guidelines 2003)
Conclusion
As per result, it can be concluded that from the cell viability assay Deferasirox and Deferasirox nanoparticle formulation showed anti-tumoral activity at tested concentration from 50 µg/mL up to 500 µg/mL whereas Kolliphor® P 188 showed higher cell viability indicating suitability of Kolliphor® P 188 as surfactant. Thus, results verified that nanoparticles loaded with Deferasirox are promising agents for targeted drug delivery in breast cancer and great potential in delivery of Deferasirox to the targeted region as an alternative to the conventional dosage form.