Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.28 no.1 Madrid ene./feb. 2013
https://dx.doi.org/10.3305/nh.2013.28.1.6055
Effects of the dietary amount and source of protein, resistance training and anabolic-androgenic steroids on body weight and lipid profile of rats
Efectos del porcentaje y fuente de proteína, del entrenamiento de fuerza y de la administración de esteroides anabolizantes sobre el peso corporal y el perfil lipídico de ratas
V. A. Aparicio1,2, C. Sánchez1, F. B. Ortega2, E. Nebot1, G. Kapravelou1, J. M. Porres1 and P. Aranda1
1Department of Physiology, School of Pharmacy, School of Sport Sciences and Institute of Nutrition and Food Technology. University of Granada. Spain.
2Department of Physical Education and Sport. School of Sport Sciences, University of Granada. Spain.
This study was supported by the project DEP2008-04376 from the Ministry of Science and Innovation and grants from the Spanish Ministry of Education (AP2009-3173) and the Ministry of Science and Innovation (BES-2009-013442).
ABSTRACT
Introduction: Dietary protein amount and source, hypertrophy resistance training (RT) and anabolic-androgenic steroids (AAS) may affect body weight and plasma and hepatic lipid profile.
Material and methods: 157 adult male Wistar rats were randomly distributed in 16 experimental groups resulting in: normal-protein (NP) or high-protein (HP) diets, whey or soy-protein diets, with or without RT and with or without AAS, for 3 months.
Results and discussion: Final body weight was lower in the RT and AAS groups compared to sedentary and non-AAS groups, respectively (all, p<0.001). Plasma total cholesterol (TC) was lower for the HP compared to the NP diets, for the whey compared to the soy-protein diets and for the AAS compared to the non-AAS groups (all, p<0.001). Plasma HDL-cholesterol was higher in the RT groups (p<0.05) but lower for the AAS groups (p<0.001), the HP and the soy-protein diets (p<0.05). Plasma triglycerides (TAG) were lower for the HP diet (p<0.001), for the RT (p=0.002) and the non-AAS groups (p=0.001). Liver TC was lower for the NP (p<0.01), for the soy-protein (p<0.05) and for the AAS groups (p<0.001). Liver TAG were lower for the whey-protein diet (p<0.001), RT and non-AAS groups (both, p<0.05). Some interactions were found, such as the greater effect of AAS on reducing body weight of rats that performed RT or ingested a HP diet (all, p<0.05). HDL-cholesterol was higher when RT was combined with HP diets (p=0.010) or non-AAS and when HP diets were combined with non-AAS (both, p<0.001). Groups that combined RT with non-AAS administration obtained the lowest hepatic TAG (p<0.05).
Conclusion: Among all the interventions tested, AAS was the factor that most negatively affected plasma and hepatic lipid profile, whereas HP diets and RT could benefit lipid profile, especially when combined.
Key words: High-protein diet. Anabolic-androgenic steroids. Soy protein. Whey protein. Resistance training. Plasma lipid profile. Hepatic lipid profile. Rats.
RESUMEN
Introducción: La cantidad y la fuente de proteína, el entrenamiento de fuerza hipertrofia (EF) y los esteroides anabolizantes androgénicos (EAA) pueden alterar el peso corporal y el perfil lipídico plasmático y hepático.
Material y métodos: 157 ratas Wistar adultas macho se distribuyeron al azar en 16 grupos experimentales del siguiente modo: dietas normoproteica (NP) o hiperproteicas (HP), proteínas de lactosuero o de soja, con y sin EF y con o sin EAA, durante un periodo experimental de 3 meses.
Resultados y discusión: El peso corporal final fue menor en los grupos con EF y EAA en comparación con los grupo sedentario y sin EAA, respectivamente (todos, p<0,001). El colesterol plasmático total (CT) fue menor en el grupo con dieta HP en comparación con las dieta NP, para las dietas de proteínas de lactosuero en comparación con las proteínas de soja, y para el grupo con EAA en comparación con el grupo sin EAA (todos, p<0,001). Las concentraciones plasmáticas de colesterol HDL fueron superiores en los grupos de EF (p<0,05) y menores en los grupos con EAA (p<0,001), y de dieta HP o con proteína de soja (p<0,05). Los triglicéridos (TAG) plasmáticos fueron menores con la dieta HP (p<0,001), el EF (p=0,002) y la no administración de EAA (p=0,001). El CT hepático fue menor en los grupos de dieta NP (p<0,01), dieta con proteínas de soja (p<0,05) y grupo de EAA (p<0,001). Los TAG hepáticos fueron menores en los grupos de dieta de proteínas de lactosuero (p<0,001), EF y sin EAA (ambos, p<0,05). Se hallaron algunas interacciones como un mayor efecto de los EAA en la reducción del peso corporal de las ratas que realizaron EF o ingirieron una dieta HP (todos, p<0,05). Las concentraciones plasmáticas de colesterol HDL fueron superiores cuando se combinó el EF con las dietas HP (p=0,010) o sin EAA y cuando las dietas HP se combinaron con el no uso de EAA (ambos, p<0,001). Finalmente, los grupos que combinaron el EF sin EAA obtuvieron los valores más bajos de TAG hepáticos (p<0,05).
Conclusión: De entre todas las intervenciones testadas, los EAA fueron el factor que más negativamente afectó al perfil lipídico plasmático y hepático, mientras que las dietas HP y el EF podrían beneficiar, en general, el perfil lipídico, especialmente cuando se combinan.
Palabras clave: Dieta hiperproteica. Esteroides anabólizantes androgénicos. Proteína de soja. Proteína de lactosuero. Entrenamiento de resistencia. Plasma perfil lipídico. Perfil lipídico hepático. Ratas.
Abbreviations
AAS: Anabolic androgenic steroids.
HDL-C: High-density lipoprotein cholesterol.
HP: High protein.
RT: Hypertrophy resistance training.
N: Nitrogen.
NP: Normal protein.
TAG: Triglycerides.
TC: Total cholesterol.
Introduction
Obesity and abnormal lipid levels contribute significantly to the risk of coronary heart disease, a major cardiovascular disease and a serious health problem1. Nutritional and dietary therapy, weight loss, exercise, and scientifically proven nutritional supplementation might be appropriate to manage dyslipidemia1-2.
High-protein (HP) diets may reduce body weight gain, fat deposition, and improve plasma lipid profile3-6. Furthermore, HP diets have shown to improve hepatic lipid profile in rodent models and in humans ingesting a high-fat diet7-9.
Several human10-11 and rodent studies4,6,12 have demonstrated the ability of whey-protein to improve body composition. Similarly, the effects of soy-protein on serum lipoproteins have been of great interest in the last decade. The new soy-based supplements may play a valuable role at reducing cardiovascular risk13-14. However, existing data are inconsistent or inadequate in supporting most of the suggested health benefits of consuming soy-protein14.
Resistance training can reduce body fat, lipids and the consequent risk of cardiovascular disease1,15-16. Furthermore, aerobic exercise17-18 and, especially resistance training, could reduce fat concentration in the human liver19 at the same time that has been shown to reduce insulin resistance in the adipose and hepatic tissue in obese rats20.
Anabolic-androgenic steroids (AAS) abuse is commonly associated with bodybuilders, weightlifters, and other athletes21. The chronic abuse of AAS results in part in extreme alterations in lipoproteins and apolipoproteins concentrations, especially in reducing HDL-cholesterol (HDL-C) and thus inducing an atherogenic profile with elevated risk of cardiovascular disease22-25.
A limitation of human studies is represented by the fact that information about the intake of AAS is generally self-reported and it is hardly possible to assess the exact dosage. Furthermore, AAS are often used in combination with other complements, drugs or substances, so it is difficult to separate their toxic effects. Hence, experimental studies conducted on animal models are mandatory given the complexity of carrying out long-term and well controlled interventional studies on this topic in human subjects. Moreover, most of the available evidence come from studies that examined the effect of specific interventions, e.g. focus on just exercise or just protein source in the diet. However, until date, the combined effect and interactions taking place between the dietary protein amount, protein source, resistance training and AAS-administration is unknown.
The present study aimed: 1) To examine the effects of HP vs normal-protein (NP) diets, whey-protein vs. soy-protein diets, hypertrophy resistance training (RT), and AAS on final body weight and plasma and hepatic lipid profile. 2) To examine potential interactions between such interventions (protein amount, protein source, RT, and AAS).
Material and methods
Animals and experimental design
A total of 160 young albino male Wistar rats were allocated into sixteen groups derived of 4 main interventions: protein amount in the diet (HP vs. NP), protein source (whey vs. soy), training (RT vs. sedentary), and AAS (with AAS vs. without AAS administration) (fig. 1). Each specific intervention (i.e. HP diet, whey-protein diet, with RT and with AAS) was developed in groups of 10 rats. The experimental period lasted 3 months.
The animals, with an initial body weight of 150±8 g, were housed from day 0 of the experiment in individual stainless steel metabolic cages designed for the separate collection and urine. The cages were located in a well-ventilated thermostatically controlled room (21±2 oC), with relative humidity ranging from 40 to 60%. A 12:12 reverse light-dark cycle (08.00-20.00 h) was implemented to allow exercise training during the day. Throughout the experimental period all rats had free access to double-distilled water and the animals consumed the four different diets (HP or NP, whey or soy protein) ad libitum. One week prior to the experimental period start, the rats were allowed to adapt to their respective diets and experimental conditions.
Body weight was measured weekly for all animals at the same time and the amount of food consumed by each rat was registered daily.
At the end of the experimental period, the animals were anaesthetized with pentobarbital and sacrificed by exsanguination by means of cannulation of the abdominal aorta. Blood was collected (with heparin as anticoagulant) and centrifuged at 3000 rpm for 15 min to separate plasma that was frozen in liquid N and stored at -80oC.
All experiments were undertaken according to Directional Guides Related to Animal Housing and Care (European Community Council, 1986)26, and all procedures were approved by the Animal Experimentation Ethics Committee of the University of Granada.
Experimental diets
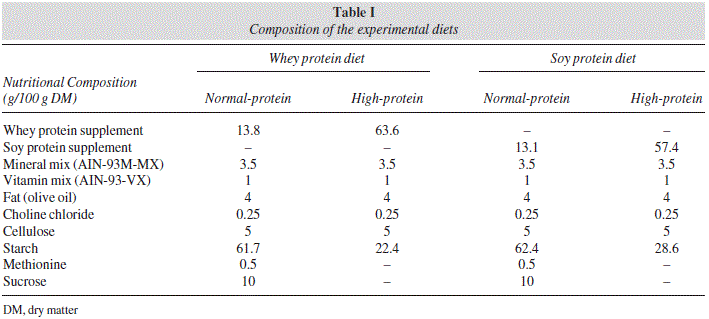
Formulation of the experimental diets is presented in table I. All diets were formulated to cover the nutrient requirements of rats following the recommendations of the American Institute of Nutrition (AIN-93M)27, with slight modifications. We have selected a 45% of protein level for the HP diet groups following previous studies in which HP diet was compared with NP diets in rats3-4628, whereas a 10% protein content was chosen for the NP diet groups. Commercial whey or soy-protein isolates were used as the only protein source since this protein source is widely available and used by sportsmen. Inclusion of 45% protein level in the diet was done at the expense of complex carbohydrates (wheat starch). Prior to the diet preparation, total protein concentration of the commercial whey and soy hydrolyzates and its distribution among the protein or non-protein fractions was measured. Total N content of the commercial whey-protein hydrolyzates was 11.8±0.6 g/100g of dry matter, which corresponds to a 73.8% of richness. Total N content of the commercial soy-protein hydrolyzate was 12.4±0.7 g/100g of dry matter, which corresponds to a 77.5% of richness.
Total protein concentration of the experimental diets was also assayed, with values of 44.3±2.1 % and 10.4±0.6% for the HP and NP, respectively, whey-protein diet, and 44.1±2.2% and 9.8±0.4% for the HP and NP, respectively, soy-protein diet. These values are adequate for our experimental design.
Chemical analyses
Total nitrogen (N) of the whey and soy-protein supplements and quadriceps was determined according to Kjeldahl's method. Crude protein was calculated as N x 6.25.
Plasma total cholesterol (TC), triglycerides (TAG) and HDL-C were measured using a HITACHI Roche p800 autoanalyzer.
Liver fat extraction was assessed by means of the Folch method with slight adaptations29. The concentration of TC and TAG in liver fat was assayed using commercial kits (Spinreact, S.A. Gerona, España).
Resistance training
The experimental groups were trained following a RT protocol in a motorized treadmill (Panlab Treadmills for 5 rats, LE 8710R) with weights in a bag tied with a cord to the tail. This type of training was chosen in order to reproduce and mimic the type of exercise performed by people interested in gaining muscle mass and strength whose usually combine high-protein diets with AAS administration. This is important for the better interpretation of the training-derived results from this study due to the fact that perhaps we would have chosen another type of exercise if our aim would have been to improve lipid profile. Therefore, our training protocol follows the established principles for human RT, involving weights, repetitions and sets to maximize gains in muscle strength30.
The training group exercised on alternate days. The animals ran at a constant speed of 35cm/s during the whole experimental period (12 weeks) in their dark phase. Prior to exercise training, animals were adapted to the treadmill on a daily basis for 1 week, first three days without weight and the last four days with 20% of their bodyweights. The training protocol used in the present study with slightly modifications has been previously developed and described by Aparicio et al.31.
Animals in the control groups were managed identically to exercising animals, with the exception of exercise training. In order to avoid a possible confounding effect due to handling in the training groups, control animals were handled weekly.
Anabolic-androgenic steroids administration
Following similar studies performed in rats, the animal received 10 mg/kg body weight of Nandrolone decanoate once a week by intramuscular injection in the gluteus (alternating the lateral side each week). This dose is comparable to the dose that has been reported as being frequently used by athletes (600 mg/week or approximately 8 mg/Kg/week)32-33. We used a commercially available nandrolone decanoate solution of 50 mg/ml (Deca-Durabolin, Organon, Oss, Netherlands).
Statistical analysis
Results are presented as mean and standard error of the mean. The effects of the dietary protein amount and source, RT and AAS on the outcome variables were analyzed by four-ways ANOVA, with the four mentioned intervention groups as fixed factors, and values of food intakes, final body weight, quadriceps N content and plasma and hepatic lipid profile as dependent variables in separate models. Two-ways interaction terms were introduced into the models to test interactions between the following variables: RT*dietary protein amount; AAS*dietary protein amount; AAS*RT; AAS*protein source, and dietary protein amount*protein source. All analyses were performed using the Statistical Package for Social Sciences (SPSS, version 16.0 for Windows; SPSS Inc., Chicago, IL), and the level of significance was set at 0.05.
Results
The effects of the dietary protein amount and source, RT and AAS-administration on final body weight, food intake, quadriceps N content, and plasma and hepatic lipid profile are shown in table II.
Final body weight, food intake and quadriceps
Nitrogen content
Final body weight was lower in the RT and AAS groups compared to the sedentary and the non-AAS groups, respectively (p<0.001). No differences on final body weight were observed depending on the dietary protein amount or source.
Along the experimental period, food consumption gradually declined in all groups, especially from the second month (data not shown). Food intake was higher in the NP compared to the HP diet groups, for the RT compared to the sedentary groups and for the AAS compared to the non-AAS groups (all, p<0.01).
We analyzed quadriceps N content since it is the musculature involved in locomotion. Quadriceps N content was higher for the HP compared to the NP diet (p=0.001), for the whey compared to the soy-protein diet, and for the RT compared to the sedentary groups (both, p<0.001).
Plasma lipid profile
Plasma TC concentrations were lower for HP compared to NP diet groups (p=0.001), for the whey compared to the soy-protein and for the AAS compared to the non-AAS groups (both, p<0.001). Plasma HDLC concentrations were lower in HP compared to the NP diet, for the soy compared to the whey-protein groups (both, p<0.05) and for the AAS compared to the non-AAS groups (p<0.001), but higher for the RT compared to the sedentary groups (p<0.05). Plasma TAG concentrations were lower for the HP compared to the NP diet groups (p<0.001), for the soy compared to the whey-protein diets (p=0.001), for the RT compared to the sedentary groups (p=0.002) and for the non-AAS compared to the AAS groups (p=0.001).
Hepatic lipid profile
Liver fat percentage was lower for the HP compared to the NP diet (p=0.002), for the sedentary compared to the RT groups (p=0.003) and for the non-AAS compared to the AAS groups (p<0.001). Liver TC concentrations were lower for the NP compared to the HP groups (p=0.007), for the soy compared to the soy-protein diets (p<0.05) and for the AAS compared to the non-AAS groups (p<0.001). Liver TAG concentrations were lower for the whey compared to the soy-protein groups (p<0.001), for the RT compared to the sedentary groups (p=0.022) and for the non-AAS compared to the AAS groups (p=0.010).
Interactions
Interactions found between the different interventions on final body weight are shown in figure 2. Groups that combined RT with AAS presented a lower final body weight (p=0.020). The same phenomena was observed when AAS or soy-protein diets were combined with HP diets (p=0.004 and p=0.032, respectively).
Interactions found on plasma lipid profile are shown in figure 3. Plasma HDL-C was the outcome implied in the majority of interactions. HDL-C concentrations were higher when RT was combined with HP diets (p=0.010) or non-AAS administration (p<0.001). In the same line, HDL-C concentrations were higher when HP diets were combined with non-AAS (p<0.001). Groups that intake the whey-protein diets in a NP diet amount also obtained the higher levels of HDL-C (p<0.001). Plasma TAG concentration were higher in NP diet groups, but especially when NP diet was combined with whey-protein diets or AAS (all, p<0.05).
Interactions found on hepatic lipid profile are shown in figure 4. Groups that combined RT with non-AAS administration obtained the lowest hepatic TAG concentrations (p<0.05). Hepatic TC was higher when whey-protein was combined with HP diets (p<0.001).
Discussion
The main findings of this study were: 1) HP diet reduced plasma TC and TAG concentrations as well as liver fat percentage. 2) Any consistent benefits on body weight loss, hepatic and plasma lipid profile have been observed derived from soy-protein instead to whey-protein consumption. 3) RT significantly reduced body weight and increased plasma HDL-C, with a more pronounced effect in the AAS-administered and HP diets groups. RT was also effective at reducing hepatic TAG. 4) AAS-administration reduced final body weight, plasma and hepatic TC, but notably decreased plasma HDL-C, promoting the decrease on TC levels. 5) Overall the results reveal that among all the interventions tested, AAS administration was the factor that most negatively affected plasma and hepatic lipid profile, whereas HP diets and RT could induce, in general, a better lipid profile, especially when combined.
Body weight and food intake
In contrast to what has been reported by some authors3-4, we have not observed a lower food intake or body weight by the HP diet consumption whereas our RT and AAS groups increased food intake and reduced body weight. Is further known that resistance training increases lean body mass and can reduce body weight1,15-16,34 and therefore we have confirmed such higher muscle mass in the present study with the higher quadriceps N content observed in the RT groups. Maybe as a direct consequence of this effect, we have observed an interaction between exercise and AAS-administration, where AAS groups with RT also displayed a lower final body weight.
Plasma lipid profile
In agreement to our results, Noakes et al.5 reported a greater reduction on plasma TAG concentrations in overweight women that consumed a HP diet when compared to a high-carbohydrate-low fat diet (NP diet), whereas also accordingly to us, weight loss was the same with both diets. Our RT groups presented lower, but not significantly, plasma TC and TAG and significantly higher HDL-C concentrations, a fact that confirms the highly contrasted effects of resistance training on lipid profile1,15-16. This better plasma lipid profile in general, could have a protective effect on cardiovascular diseases1,35.
Some studies have documented potential safety concerns on increased consumption of soy products14,36. We cannot confirm the existence of lower TC concentrations after the soy-protein diet consumption under our experimental design. In fact, HDL-C was lower for the soy-protein compared to the whey-protein diet. However, TAG concentrations were lower in the soy-protein fed groups. To note is that soy-protein appears to have demonstrated effect only on reducing LDL-C14. Moreover, when studying the effects of soy-protein, the exact combination of active ingredients in soy products need to be identified36. Choquette et al.37 aimed to analyze the combined effect of exercise and isoflavones in overweight-to-obese postmenopausal women (we do not know the specific isoflavones content in our diet). The main effects of exercise were observed for total fat mass, however, and in a similar way to what has been reported in our study, no interactions on lipid profile were observed between soy-protein and RT.
The effects of AAS-administration on plasma lipid profile have been studied in male body builders who received a weekly intramuscular injection of nandrolone-decanoate (100 mg) or placebo for 8 weeks in a double blind way. AAS induced a ~26% decrease in HDL-C24. Frisch and Sumida25, studied whether compromised serum lipoprotein concentrations would be evident in rats receiving testosterone injections over the time course of 7 weeks. No significant differences were observed between groups for any serum lipid parameters concentration. However, at week 7, serum HDL-C was significantly lower in the testosterone treated rats, compared with control animals. The authors concluded that lipoprotein profile is not altered until week 7 (our study has been performed during 12 weeks). In the study of Bonetti et al.23 20 male body builders, voluntarily starting AAS-administration,
were followed every 6 months over 2 years. The most important long-term adverse effects were lower fertility and newly the impairment of lipid profile (especially HDL-C), associated with an increased cardiovascular risk.
Hepatic lipid profile
Despite plasma TC was lower for HP groups, hepatic TC did not follow the same trend. A possible explanation for this lack of relationship between hepatic and plasma lipid profile could be that some fatty acids are usually present in different distribution in the liver38.
Recently, Bortolotti et al.39 evaluated the effects of a whey-protein supplementation for 4 weeks on intrahepatocellular lipids and fasting plasma TAG in obese non diabetic women. Whey-protein decreased intrahepatocellular lipids by ~21%, fasting total TAG by ~15%, and TC by ~7%. The authors concluded that whey-protein reduces hepatic steatosis and improves plasma lipid profile in obese non diabetic patients, without adverse effects on glucose tolerance or creatinine clearance39. We have also obtained lower values of TAG among the whey-protein groups but we cannot confirm a significant hepatic TC reduction when compared to the soy-protein groups, which had slighty lower TC.
Weight loss remains the most common therapy advocated for reducing hepatic lipids in obesity and nonalcoholic fatty liver disease, whereas results regarding the effects of exercise on hepatic lipid profile are still scarce or not conclusive. Some studies have reported that hepatic TAG from trained animals contain more saturated and less unsaturated (monounsaturated as well as polyunsaturated) fatty acids than control groups without exercise19,40. We have observed a very significant hepatic TAG reduction in our trained groups, especially when were combined with non-AAS administration. This concurs with the study by Johnson et al.17, whose observed that hepatic TAG concentrations were reduced by 21% after 4 weeks of aerobic cycling exercise in obese women. The authors concluded that regular aerobic exercise reduces hepatic lipids in obesity even in the absence of body weight reduction. On the other hand, Petridou et al.18 examined the effects of 8 weeks of exercise training on the fatty acid composition of phospholipids and TAG in rat liver. The fatty acid composition of liver phospholipids changed with training whereas no significant differences in the fatty acid profile of hepatic TAG were found.
Hepatic TAG concentrations were higher with AAS-administration, what emphasizes the adverse effect of AAS on lipid profile. A recent study has concluded that AAS could be a possible new risk factor for toxicant-associated steatohepatitis or toxicant-associated fatty liver disease development. Moreover, all cases were asymptomatic and in this type of fatty liver disease, the individuals had a low body fat mass and they did not present insulin resistance41.
Limitation and strengths
Some limitations need to be mentioned: First, the current physiological results obtained in rodents must be confirmed in human subjects and cannot be extrapolated directly to the potential effects in humans. Second, to measure some additional lipoproteins and LDL-C would have been of interest for the interpretation of the results. On the other hand, this study involved an important number of rats, allocated in different groups so that the main effects of HP diet, RT, the protein source and AAS-administration and the interactions taking place between them, provided a good opportunity to comprehensively investigate how these lifestyle factors and behaviors can influence dyslipidemia and the risk of coronary heart disease.
Conclusion
The AAS administration was the factor that most negatively influenced plasma and hepatic lipid profile. HP diet showed a moderate positive effect on plasma lipid profile. Soy-protein did not appear to be especially effective when compared to whey-protein at promoting weight loss or improving plasma and hepatic lipid profile. The RT performed in the present study significantly reduced body weight and increased plasma HDL-C, with a more pronounced effect in the AAS and HP diets groups. Finally, AAS reduced final body weight, plasma and hepatic TC, but notably decreased plasma HDL-C, which could be the reason of the lower TC observed. Overall the results reveal that among all the interventions tested, AAS administration was the factor that most negatively affected plasma and hepatic lipid profile, whereas HP diets and RT could induce, in general, a better lipid profile, especially when combined.
Acknowledgments
The authors gratefully to all the members from the Department of Physiology for their collaboration, especially to Lucía Bustos and the rest of people involved in the field work for their efforts and great enthusiasm.
Competing interest
The authors declare that they have no competing interests
References
1. Houston MC, Fazio S, Chilton FH, et al. Nonpharmacologic treatment of dyslipidemia. Prog Cardiovasc Dis 2009; 52: 61-94. [ Links ]
2. Detection TRotNCEPNEPo. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143-421. [ Links ]
3. Lacroix M, Gaudichon C, Martin A, et al. A long-term highprotein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 2004; 287: R934-42. [ Links ]
4. Pichon L, Potier M, Tome D, et al. High-protein diets containing different milk protein fractions differently influence energy intake and adiposity in the rat. Br J Nutr 2008; 99: 739-48. [ Links ]
5. Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr 2005; 81: 1298-306. [ Links ]
6. Belobrajdic DP, McIntosh GH, Owens JA. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J Nutr 2004; 134: 1454-8. [ Links ]
7. Bortolotti M, Kreis R, Debard C, et al. High protein intake reduces intrahepatocellular lipid deposition in humans. Am J Clin Nutr 2009; 90: 1002-10. [ Links ]
8. Gudbrandsen OA, Wergedahl H, Liaset B, Espe M, Mork S, Berge RK. Dietary single cell protein reduces fatty liver in obese Zucker rats. Br J Nutr 2008; 100: 776-85. [ Links ]
9. Pichon L, Huneau JF, Fromentin G, Tome D. A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats. J Nutr 2006; 136: 1256-60. [ Links ]
10. Cribb PJ, Williams AD, Stathis CG, Carey MF, Hayes A. Effects of whey isolate, creatine, and resistance training on muscle hypertrophy. Med Sci Sports Exerc 2007; 39: 298-307. [ Links ]
11. Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care 2008; 11: 40-4. [ Links ]
12. Baer DJ, Stote KS, Paul DR, Harris GK, Rumpier WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr 2011; 141: 1489-94. [ Links ]
13. Hermansen K, Dinesen B, Hoie LH, Morgenstern E, Gruenwald J. Effects of soy and other natural products on LDL: HDL ratio and other lipid parameters: a literature review. Adv Ther 2003; 20: 50-78. [ Links ]
14. Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr 2008; 138: 1244S-9S. [ Links ]
15. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006; 84: 475-82. [ Links ]
16. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007; 116: 572-84. [ Links ]
17. Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009; 50: 1105-12. [ Links ]
18. Petridou A, Nikolaidis MG, Matsakas A, Schulz T, Michna H, Mougios V. Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue. Eur J Appl Physiol 2005; 94: 84-92. [ Links ]
19. Magkos F. Exercise and fat accumulation in the human liver. Curr Opin Lipidol 2010; 21: 507-17. [ Links ]
20. da Luz G, Frederico MJ, da Silva S, et al. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur J Appl Physiol 2011; 111: 2015-23. [ Links ]
21. Turillazzi E, Perilli G, Di Paolo M, Neri M, Riezzo I, Fineschi V. Side Effects of AAS Abuse: An Overview. Mini Rev Med Chem 2011; 11: 374-89. [ Links ]
22. Frohlich J, Kullmer T, Urhausen A, Bergmann R, Kindermann W. Lipid profile of body builders with and without self-administration of anabolic steroids. Eur J Appl Physiol Occup Physiol 1989; 59: 98-103. [ Links ]
23. Bonetti A, Tirelli F, Catapano A, et al. Side effects of anabolic androgenic steroids abuse. Int J Sports Med 2008; 29: 679-87. [ Links ]
24. Kuipers H, Wijnen JA, Hartgens F, Willems SM. Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders. Int J Sports Med 1991; 12: 413-8. [ Links ]
25. Frisch F, Sumida KD. Temporal effects of testosterone propionate injections on serum lipoprotein concentrations in rats. Med Sci Sports Exerc 1999; 31: 664-9. [ Links ]
26. Estoppey-Stojanovski L. Position of the Council of Europe on the protection of animals. Dev Biol Stand 1986; 64: 3-5. [ Links ]
27. Reeves PG, Nielsen FH, Fahey GC, Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939-51. [ Links ]
28. Amanzadeh J, Gitomer WL, Zerwekh JE, et al. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int. 2003; 64: 2142-9. [ Links ]
29. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497-509. [ Links ]
30. de Salles BF, Simao R, Miranda F, Novaes Jda S, Lemos A, Willardson JM. Rest interval between sets in strength training. Sports Med 2009; 39: 765-77. [ Links ]
31. Aparicio VA, Nebot E, Porres JM, et al. Effects of high-whey-protein intake and resistance training on renal, bone and metabolic parameters in rats. Br J Nutr 2010: 1-10. [ Links ]
32. Chaves EA, Pereira-Junior PP, Fortunato RS, et al. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol 2006; 99:223-30. [ Links ]
33. Cunha TS, Tanno AP, Costa Sampaio Moura MJ, Marcondes FK. Influence of high-intensity exercise training and anabolic androgenic steroid treatment on rat tissue glycogen content. Life Sci 2005; 77: 1030-43. [ Links ]
34. Tremblay A, Despres JP, Bouchard C. The effects of exercise-training on energy balance and adipose tissue morphology and metabolism. Sports Med 1985; 2: 223-33. [ Links ]
35. Bianchi C, Penno G, Romero F, Del Prato S, Miccoli R. Treating the metabolic syndrome. Expert Rev Cardiovasc Ther 2007; 5: 491-506. [ Links ]
36. Kerckhoffs DA, Brouns F, Hornstra G, Mensink RP. Effects on the human serum lipoprotein profile of beta-glucan, soy protein and isoflavones, plant sterols and stanols, garlic and tocotrienols. J Nutr. 2002; 132: 2494-505. [ Links ]
37. Choquette S, Riesco E, Cormier E, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr 2011; 105: 1199-209. [ Links ]
38. Kotronen A, Seppanen-Laakso T, Westerbacka J, et al. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity (Silver Spring). 2010; 18: 937-44. [ Links ]
39. Bortolotti M, Maiolo E, Corazza M, et al. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin Nutr 2011; 30: 494-8. [ Links ]
40. Simko V, Ondreicka R, Chorvathova V, Bobek P. Effect of long-term physical exercise on bile sterols, fecal fat and fatty acid metabolism in rats. J Nutr 1970; 100: 1331-9. [ Links ]
41. Schwingel PA, Cotrim HP, Salles BR, et al. Anabolic-androgenic steroids: a possible new risk factor of toxicant-associated fatty liver disease. Liver Int 2011; 31: 348-53. [ Links ]
![]() Correspondence:
Correspondence:
Virginia A. Aparicio García-Molina.
Department of Physiology.
School of Pharmacy.
University of Granada.
Campus Universitario de Cartuja, s/n.
18071 Granada (España).
E-mail: virginiaparicio@ugr.es
Recibido: 13-VII-2012.
Aceptado: 26-VIII-2012.