INTRODUCTION
Breast cancer is a public health problem worldwide 1 due to its high incidence as well as the toxicity of antineoplastic treatments that affect nutritional status as well as the quality of life 2. Moreover, patients with cancer frequently present alterations in body composition, such as loss of muscle mass and increased adipose tissue, which, in turn, relate to an increased risk of developing cardiometabolic alterations 8,9. These conditions are also considered to be secondary to the chronic inflammation 10,11. Likewise, some studies consider that the affection of body composition and the increase of systemic inflammatory mediators cause an increase in toxicity secondary to chemotherapy, thus yielding a vicious circle that negatively affects the quality of life of patients presenting an increase in symptomatology associated with chemotherapy as fatigue, pain, anxiety and depression, as well as sleep disturbances and an increased risk of early recurrence of the disease 3,6,12. Hence, co-adjuvant therapies are of profound interest.
In line with this, some studies have shown that omega 3 polyunsaturated fatty acids (PUFA Ω-3) may attenuate such side effects and, in addition, help to maintain body weight and preserve muscle mass 3,4,5,6. These benefits have been attributed to their anti-inflammatory effects, including an increase in circulating adiponectin and other anti-inflammatory molecules, concomitant to the suppression of proinflammatory pathways such as that mediated by nuclear factor-kappa B (NF-κB) 3,6,7. Previous studies in breast and esophageal cancer have reported that supplementation with PUFA Ω-3 during chemotherapy improves the quality of life of these patients by improving anemia, thrombocytopenia and reducing gastrointestinal toxicity (mucositis, stomatitis and diarrhea) 13,14. In addition, supplementation with 2.5 g PUFA Ω-3 during chemotherapy in patients with lung cancer showed to preserve body weight and skeletal muscle 15.
In breast cancer, neoadjuvant chemotherapy (NeoCT) is the first-line treatment in patients with locally advanced disease 16. NeoCT is administered before a radical treatment (surgical and/or radiotherapy) in order to control the systemic disease, reduce the size of the tumor and, in some cases, achieve a complete pathological response of the tumor. This improves locoregional control, disease-free periods and the overall survival of patients 17. However, nowadays there are no studies analyzing the toxic effect of NeoCT in patients with locally advanced breast cancer (LABC).
Based on these observations, we hypothesized that the supplementation with PUFA Ω-3 may decrease the toxicity secondary to chemotherapy during the NeoCT treatment in patients with LABC.
MATERIALS AND METHODS
STUDY DESIGN
A randomized, double-blinded, placebo-controlled trial was conducted during the six months of NeoCT of 53 women with LABC. They were randomized into two groups: 27 women for the PUFA Ω-3 group and 26 for the placebo group. Inclusion criteria were: women aged 18-80 years, with a diagnosis of LABC confirmed by histopathology, in a clinical stage IIA-III B. Exclusion criteria were: patients who received chemotherapy different from anthracyclines-taxanes, uncontrolled chronic diseases (chronic heart failure, renal insufficiency, hypertension, diabetes mellitus), known allergy to fish or fish oil, alterations of the digestive tract that would prevent the intake of the supplement, consumption of other supplements with PUFA Ω-3, and BMI < 18 kg/m2.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki. It was approved by the Universidad Anáhuac México IRB and the Ethics Committee of the National Commission of Scientific Research of the National Institute of Cancerology (reg. 015/035/IMO CEI/971). Each participant provided written informed consent.
TREATMENT
All participants received NeoCT: four cycles of adriamycin and cyclophosphamide, followed by 12 weeks of paclitaxel +/- trastuzumab. Patients also received an oral supplement with PUFA Ω-3 derived from fish oil in gel capsules, daily dosed at 2.4 g (four capsules) in a 2:1 ratio of DHA/EPA, or a placebo (sunflower oil) during the six months of chemotherapy. Capsules were identical in appearance (Alta Tecnología en Alimentos Funcionales S.A. de C.V., Mexico) and in accordance with the Good Manufacturing Practices for food. Adherence was assessed every month by counting the leftover capsules of the previous month; patients with compliance < 90% were excluded from analysis.
TOXICITY EVALUATION
Adverse events were evaluated by the attending physician, three weeks after the beginning of NeoCT, in accordance with the CTCAE (version 4.03, June 2010). Acute events, unscheduled hospital admissions and delayed chemotherapy were obtained from medical records. The patient's perspective of some symptoms (including pain, fatigue, drowsiness, nausea, appetite, dyspnea, anxiety, insomnia, malaise, and xerostomia) was evaluated with the Edmonton Symptom Assessment System (ESAS, Spanish version) 18,19.
BODY COMPOSITION EVALUATION
Weight and body composition (body fat mass, fat-free mass, skeletal muscle, body mass index [BMI]) were evaluated by bioelectrical impedance (Inbody 720; Biospace, Ltd., Seoul Korea), using an eight-point tetrapolar tactile electrode system. Measurements were obtained at the beginning of the study, and again at three and six months 20).
METABOLIC EVALUATION
Blood samples were obtained at the beginning of the study (prior to any treatment), three and six months after therapy. Serum glucose, lipid profile (total cholesterol, HDL and LDL cholesterol and triglycerides), insulin, glycated hemoglobin (HbA1c), liver function tests, urea and creatinine were determined by immunoassay (Beckman Coulter, California, USA). Insulin resistance (IR) was estimated using the homeostatic model (HOMA-IR) 21.
STATISTICAL ANALYSIS
The Kolmogorov-Smirnov test was used to evaluate normality of the data. Demographic variables are presented as descriptive statistics. The differences between PUFA Ω-3 and the placebo groups were compared using Student's t test for independent groups. Repeated measures ANOVA was used to evaluate intra-group changes. Proportions were evaluated using the Fisher's exact test. Statistical significance was considered when p < 0.05.
RESULTS
A total of 81 patients were screened for this study; 53 were included and randomized: 27 women for PUFA Ω-3 and 26 for placebo supplementation (Fig. 1). Recruitment was carried out from March 2015 to January 2016, whereas clinical follow-up was completed in September 2017.
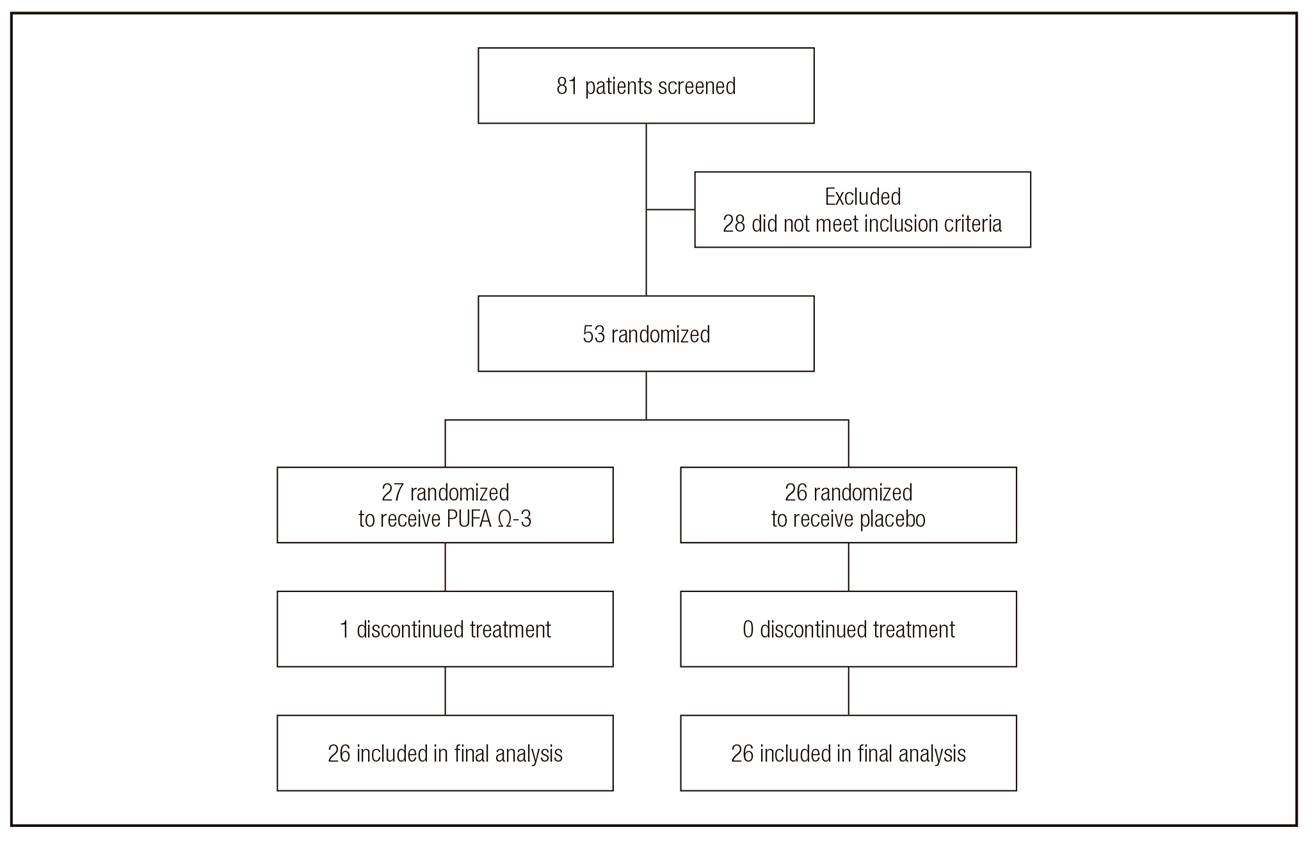
Figure 1. CONSORT. The algorithm of allocation of study subjects to analyzed groups. Fifty-three women diagnosed with locally advanced breast cancer (LABC) treated with neoadjuvant chemotherapy and randomized to receive a capsule supplement with omega 3 polyunsaturated fatty acids (PUFA Ω-3) or placebo. Only one patient discontinued the treatment (omega 3 supplementation) because of lack of interest in supplementation.
Mean age of the patients was 50.7 ± 2.1 (range 28-72) and 49.5 ± 2.1 years (range 29-68) in the groups of PUFA Ω-3 and placebo, respectively (p ns). Comorbid conditions were registered in 19 patients of the sample (36.5%): 13 (25%) patients with diabetes mellitus (DM2) and nine patients (17.3%) with systemic arterial hypertension (HAS). Twenty-seven patients (52%) were premenopausal. Four (7.7%) patients were classified as stage IIA, 20 (38.5%) patients as IIB, 22 (42.3%) patients as IIIA and six (11.5%) patients as IIIB. Infiltrating ductal carcinoma was the most prevalent histological subtype, presented in 46 (90%) patients. Clinical characteristics of the patients are shown in Table I.
Table I. Enrolment and baseline characteristics of studied subjects

Data reported as mean ± standard error (SEM). PUFA Ω-3: omega-3 polyunsaturated fatty acids.
ADVERSE EVENTS
The most common data of toxicity secondary to the administration of chemotherapy were hematological alterations: leukopenia, neutropenia and anemia were similar in both groups and there was no significant difference between them. As a consequence of hematological toxicity, seven patients (27%) of the PUFA Ω-3 and nine patients (35%) of the placebo group had a delay in the administration of treatment with adriamycin-cyclophosphamide and, during the administration of treatment with paclitaxel, five (20%) patients from the PUFA Ω-3 group and seven (28%) patients from the placebo group postponed their administration.
No significant differences in symptoms related to gastrointestinal toxicity such as nausea, vomiting, diarrhea and mucositis were found between groups, neither in fatigue nor neuropathy. These results are shown in Table II.
EDMONTON SCALE ASSESSMENT
Both the PUFA Ω-3 and placebo groups presented a similar significant increase at three and six months in the fatigue scales (p = 0.001), nausea (p = 0.031), drowsiness (p = 0.001), appetite (p = 0.002) and dyspnea (p = 0.033); however, no significant differences were found between groups. Pain, discouragement and anxiety did not present statistically significant changes during the time (three and six months of the intervention). Xerostomia scale showed a significant decrease in the intensity of this symptom over time (three and six months) in the group supplemented with PUFA Ω-3 (p = 0.0001). This symptom also showed a significant decrease in the group supplemented with PUFA Ω-3 (p = 0.032), compared with the placebo one.
BODY WEIGHT AND COMPOSITION
At the beginning of the study, 77.7% of the patients were overweight. Body weight and BMI showed no statistically significant difference during the time the study was carried out or between the groups. Average weight loss for the group supplemented with PUFA Ω-3 was 1.8 kg, while the group that received the placebo presented a weight increase of 0.5 kg (Table III).
Table III. Body composition

Data reported as mean ± standard error (ESM). PUFA Ω-3: omega 3 polyunsaturated fatty acids; CI: 95% confidence interval. Analyzed by the ANOVA test of repeated measures. Statistically significant difference at p < 0.05).
The skeletal muscle index (SMI) showed a significant (p = 0.02) increase over time in both groups but no difference was found when compared between groups. Fat free mass (FFM) exhibited the same phenomenon. For its side, body fat showed a significant reduction during the time of measurements at three and six months in the group supplemented with PUFA Ω-3 (p = 0.02), while in the patients receiving placebo it did not present changes. These results are shown in Table III.
CARDIOMETABOLIC PROFILE
Carbohydrate metabolism (serum glucose, insulin, HOMA-IR, HbA1c) was not affected by NeoCT over time in any group. In the lipid profile, a significant increase in the concentration of triglycerides (TG) at three and six months (p = 0.0001) was found in both groups. Similarly, cholesterolemia was significantly higher at three and six months (p = 0.04), but no differences were found when comparing the effect of the supplementation with PUFA Ω-3 vs placebo. On the opposite, HDL presented a significant reduction in both groups (p = 0.0001); this effect was not significant when it was compared between the two groups.
Serum albumin showed a reduction that was statistically significant (p = 0.0001), however, this effect was not significant in relation to PUFA Ω-3 or placebo groups. Also, alanine aminotransferase (ALT) was measured to evaluate liver damage. The two groups show an increase considered to be still normal, which was significant at three and six months (p = 0.002). Protein degradation was assessed by the concentration of urea nitrogen (BUN). The changes were not significant over time in any group.
DISCUSSION
In the present study, the effects of oral supplementation with PUFA Ω-3 (DHA and EPA) on the toxic effects of NeoCT in patients with LABC were investigated.
Previous studies evaluated treatment efficacy and indirect toxicity with the quality of life scales and with the CTCAE (version 4.03). The present study is the first to consider the perception of some symptoms associated with the toxicity of the patient with the Edmonton scale 18. In general, no statistically significant effects were observed in toxicity; however, the patients who received the supplement with PUFA Ω-3 presented improvement in the scale of xerostomia. This result could support the one reported by a previous study, where it was found that supplementation with PUFA Ω-3 in patients with esophageal cancer and treated with NeoCT decreases mucosal damage induced by chemotherapy 14. The protective effect of PUFA Ω-3 was related to the reduction of systemic inflammation, since PUFA Ω-3 modulates the production of proinflammatory cytokines and the production of anti-inflammatory eicosanoids and, indirectly, promotes the decrease in the production of TNF-alpha and IL-6 23,24.
Although PUFA Ω-3 is rapidly incorporated into blood cells during consumption, no improvement in hematological toxicity was observed 14. The results of this study indicate that supplementation with PUFA Ω-3 does not protect against the hematological toxicity induced by chemotherapy. There are studies reporting that a combined chemotherapy treatment (as the one used in the present study) is more severe as compared to a monotherapy and the hematological toxicity (neutropenia and leukopenia) is substantially higher in treatments with polychemotherapy 6. The results of the present study show no positive effect with PUFA Ω-3 supplementation, and this may be due to the therapy that was used, namely, adriamicin-cyclophosphamide and paclitaxel +/- trastuzumab (polychemotherapy, 12 weeks), whose side effects were surely more severe. Interestingly, and contrary to this result, the group of Miyata et al. (2012) showed that PUFA Ω-3 in NeoCT have positive effects on hematological toxicity in a cohort of patients with esophageal cancer treated with monotherapy (paclitaxel) 25.
From the clinical and epidemiological point of view, body composition plays an important role to prevent or treat different diseases. In a meta-analysis where the effect of supplementation with PUFA Ω-3 during chemotherapy on body composition was evaluated, Buckinx et al. (2015) 26 reported that the most common benefit is to increase or preserve body weight 6. The results of this study show that most of the patients had a decrease in muscle mass and a high percentage of body fat (sarcopenic obesity) before starting NeoCT. However, during the administration of chemotherapy, both groups showed an increase that was significant in the fat-free mass and the skeletal muscle index. In addition, the patients of the group supplemented with PUFA Ω-3 also had a greater reduction of mass body fat, while the placebo group showed a slight increase. There were no new cases of sarcopenic obesity during the whole treatment with chemotherapy. The results observed differ from those reported by other authors in different studies of breast cancer in advanced stages, as they report a greater amount of patients with obesity and sarcopenia during treatment 10,27. It is possible that the difference in the results is associated with the clinical stage of the disease.
In line with this, Ayca et al. (2018) reported that supplementation with DHA during chemotherapy using only one drug did not cause significant changes in inflammatory mediators or body composition of overweight and obese patients. They reported that, probably, no significant changes in body composition were found due to the differences between the study population, the dose and the duration of supplementation with DHA 3. In addition, Hames (2017) reported that, after administration of PUFA Ω-3 for six months at high doses, it increased significantly in plasma and adipose tissue. However, the changes did not show beneficial effects on markers of inflammation and in the number or subtype of macrophages present in the adipose tissue. In this study, they reported that possibly no positive effects were found due to the presence of obesity, overweight and the insulin resistance present in the patients 28. In the same way, Kratz et al. (2013) showed that supplementation with PUFA Ω-3 for 14 weeks in overweight and obese people had no effect on the expression of genes, which regulate the production of inflammatory mediators, including TNF-α in the adipose tissue 29. According to the results of these studies, it should be considered that one of the possible causes for which no statistically significant changes were found, in body composition and chemotoxicity, is the type of patients included in the present study, who were also overweight, obese and with sarcopenia before starting NeoCT.
Some studies have shown that PUFA Ω-3 reduce triglycerides. This effect has been related mainly to the decrease in the production of very low-density lipoproteins (VLDL) and, secondly, to the increase in HDL 30. However, the results of this research work show an opposite effect, since there was a significant increase in triglycerides and a reduction in HDL cholesterol in the two groups of patients. These results support what Sharma et al., who investigated changes in lipid metabolism during chemotherapy in patients with breast cancer in stage IA, IIA, IIB and IIIC, reported in their study (2016). Their results showed that the administration of doxorubicin decreases HDL and the administration of paclitaxel causes a significant increase in VLDL and triglycerides.
On the other hand, some studies suggest that the administration of PUFA Ω-3 increases insulin sensitivity, predominantly by reducing body fat mass 31,32,33. In the present study, the group of patients supplemented with PUFA Ω-3 presented a significant reduction in body fat mass; however, this change did not increase sensitivity to insulin. These findings support what has been reported by other studies, where association between supplementation with PUFA Ω-3 and insulin sensitivity was not demonstrated 28,33. It appears that the effects of PUFA Ω-3 may vary according to the severity of the disease and some characteristics of patients, such as excessive weight and insulin resistance 34.
Finally, we must mention that the lack of effect of PUFA Ω-3 in the studied patients could also be the result of other factors such as dose, type of cancer, administration scheme or even the number of patients studied. Although these parameters were used according to other similar studies reported in lung cancer, esophageal cancer, metastatic breast cancer and women at high risk of breast cancer, these may not be adequate for the pathology and the type of patients studied in the present work 13,15,35,36.
The results of this study do not support the hypothesis that PUFAs Ω-3 improve the adverse effects of NeoCT in women with LABC.
CONCLUSIONS
The results of this study indicate that supplementation with PUFA Ω-3 during neoadjuvant therapy in patients with LABC only reduces the symptoms of xerostomia. No significant differences were found in body composition, so we concluded that supplementation does not contribute to preserve weight and body composition during treatment with NeoCT. Hematological toxicity also did not show significant changes induced by PUFA Ω-3. However, it is important to consider that these results do not exclude the protective role of PUFA Ω-3 and its use as coadjuvant therapy in patients with other types of cancer. Future studies should focus on confirming these results and design suitable clinical trials to determine the proper dose and duration of PUFA Ω-3 supplementation. In addition, inflammatory markers should be assessed to evaluate the anti-inflammatory benefit of the administration of PUFA Ω-3 during the treatment of NeoCT.
STUDY LIMITATIONS
The patients included are subjected to a rigorous scrutiny that qualifies them to receive or not care in the hospital, so from the beginning, they are patients already screened, for a better profile selection, and this could bias the results, not showing the potential benefit in a "common and current" patient.















