INTRODUCTION
Colorectal cancer (CRC) is the fourth most common cancer in the world 1. The etiology of CRC is known to be multifactorial, responding to family, environmental and dietary agents 2,3,4 putative preneoplastic lesions, are early morphological changes induced by the colon carcinogen azoxymethane (AOM. It is well established that inflammation predisposes the organism to progression of CRC. Because of this, patients with inflammatory bowel disease (IBD) have an increased risk of developing CRC 5,6. Although the molecular pathogenesis of CRC associated with inflammation is not yet fully understood, significant advances have been made in studying models of CRC initiation in the rat 4,7.
Inducing CRC by azoxymethane (AOM) and sodium dextran sulfate (DSS) is a reproducible and relatively inexpensive model for initiation promotion that uses chemical induction of DNA damage, followed by repeated cycles by inflammatory agents 4 such as Crohn's disease (CD. AOM is a pro-carcinogen that is metabolized in the liver by cytochrome p450, CYP2E1 isoform, converting it into highly reactive alkylating species that induces O6-methyl-guanine abducts in DNA, which result in G → A transitions. After excretion in the bile, the methyl group is captured by the epithelium of the colon and induces mutagenesis in the KRAS oncogene and in the tumor suppressor gene CTNNB1 8 the underlying mechanisms remain to be elucidated. Inflammation can indeed provide initiating and promoting stimuli and mediators, generating a tumour-prone microenvironment. Many murine models of sporadic and inflammation-related colon carcinogenesis have been developed in the last decade, including chemically induced CRC models, genetically engineered mouse models, and xenoplants. Among the chemically induced CRC models, the combination of a single hit of azoxymethane (AOM. In addition, administration of AOM has been reported to generate kidney and liver tumors as a side effect 9,10. DSS is a polysaccharide that dissolves in drinking water and can generate damage in the colon epithelium, inducing inflammation that mimics some of the characteristics of inflammatory bowel disease (IBD). The combination of AOM and DSS provides a safe model for generating aberrant crypt foci (ACF), which are considered putative preneoplastic lesions because they share morphological, biochemical and other characteristics with tumors. These include a comparable increase in cell proliferation, the expression of antigens associated with tumors, and cellular dysplasia 2putative preneoplastic lesions, are early morphological changes induced by the colon carcinogen azoxymethane (AOM.
Although the model has been used in the past, there are no studies to date that relate nutritional markers to the formation of ACF, even though nutritional intervention is known to be an effective and promising complementary strategy for controlling the incidence of ACF 11,12,13. Similarly, there are no studies that report on modifications over time, for example, sub-chronic for 16 weeks and chronic for 32 weeks.
For this reason, it would be useful to develop a model of aberrant crypt foci induction by AOM/DSS, in order to determine its effect on nutritional markers: body weight gain, consumption of food and liquids, weight of organs at sacrifice, blood biochemistry for lipid profile, glucose, markers of renal and hepatic toxicity, and histopathological analysis of organs responsible for metabolism and excretion. All this can be observed in sub-chronic and chronic stages in Sprague Dawley rats.
MATERIALS AND METHODS
EXPERIMENTAL MODEL
Comparative longitudinal experimental study. Twenty-four-week old male Sprague Dawley rats (~145 ± 5 g)
were obtained from the UNAM Neurobiology Institute in Juriquilla, México. The animals were maintained with Lab diet 501 food (PMI International LLC, Brentwood, MO, USA) and tap water ad libitum, under a circadian cycle of 12 h light and 12 h dark at 25 °C. The care and treatment of animals were in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Academy of Science 14 and approved by the Bioethics Committee of the Faculty of Natural Sciences (23-1FCN2016). After one week of acclimatization, the rats were randomly placed into two groups (n = 12): 1) Control (basal diet + tap water); 2) AOM/DSS (basal diet + tap water + AOM/DSS). AOM was administered by subcutaneous injection of 10 mg/kg body weight (AOM dissolved in 0.5 mL of 0.9% physiological saline solution) in weeks 2 and 3 of the experiment, followed by 7 days of 2% DSS (average Mw > 500,000) in their daily drinking water as a ACF promoter. Half of the animals were sacrificed after 16 weeks (sub-chronic stage) and the rest after 32 weeks (chronic stage) (Fig. 1). After the sacrifice by decapitation, colon, liver and kidneys were dissected and fixed in 10% buffered formalin. Lesion classification was performed by an expert veterinarian pathologist.
ORGAN WEIGHTS
After decapitation, the liver, kidneys, heart, thymus, spleen, pancreas, small intestine and colon were dissected. All organs were weighed on an analytical balance and the intestines were measured longitudinally. The weights of the organs were corrected by the weight of the animal at the time of sacrifice and analyzed based on the control group.
ABERRANT CRYPT FOCI ANALYSIS
The distal colon was taken, washed in physiological saline solution, opened longitudinally, cut approximately 4 cm from the anus and fixed in 10% buffered formalin for at least 24 h. After fixation, the colon was stained with 0.2% methylene blue and examined under a microscope (10X). ACF was distinguished using Bird's method 15,16,17.
HEMATOLOGICAL ANALYSIS
Blood samples were taken immediately after the decapitation and recovered in tubes with anticoagulant. They were processed immediately to determine hematic biometrics in a Kx-21N - Hematology Analyzer (Sysmex America, Inc. One Nelson C. White Pkwy U.S.A.). For blood chemistries, the samples were recovered in tubes with separating gel. Subsequently, the samples were centrifuged (Hettich Zentrifugen eba 20; Andreas Hettich GmbH & Co. KG, Germany) for 10 min at 112 RCF and the serum recovered and analyzed in a Spin 120 (Spinreact SAUCtra Inc. Sta. Coloma, 7 17176 St. Esteve d'en Bas Girona, Spain). The analyses were carried out at the Carlos Alcocer Cuarón Nutrition Clinic of the Department of Natural Sciences at the Autonomous University of Querétaro.
HISTOPATHOLOGY ANALYSES
The tissues were dehydrated and embedded in paraffin blocks using a Histoquinete (Leica TP1020, Leica Camera AG, Wetzlar, Germany). Sections of 4 µm thickness were cut from each block and mounted on gelatin-coated slides in hot water. Tissues were rehydrated and stained with hematoxylin and eosin, then dehydrated again and sealed with Entellan solution and a coverslip. The analyses were performed under a microscope (Zeiss Axio Vert-A1, Carl Zeiss Inc., Oberkochen, Germany) at 10X and 40X magnification based on the morphology of normal colon, kidney and liver tissues 18.
RESULTS
Body weight gain and food consumption were not affected (p > 0.05), but interim trends were observed: food consumption and body weight gain decreased at the time of the first injection of AOM, but recovered by the end of the administration of AOM and DSS in both stages. An increase in liquid consumption by the animals in the AOM/DSS group was observed in comparison to the sub-chronic control group (42.2 ± 6.6 vs. 45.3 ± 7.8; p < 0.05) and chronic group (42.6 ± 5.2 vs. 44.18 ± 5.7; p < 0.05) (Fig. 2).
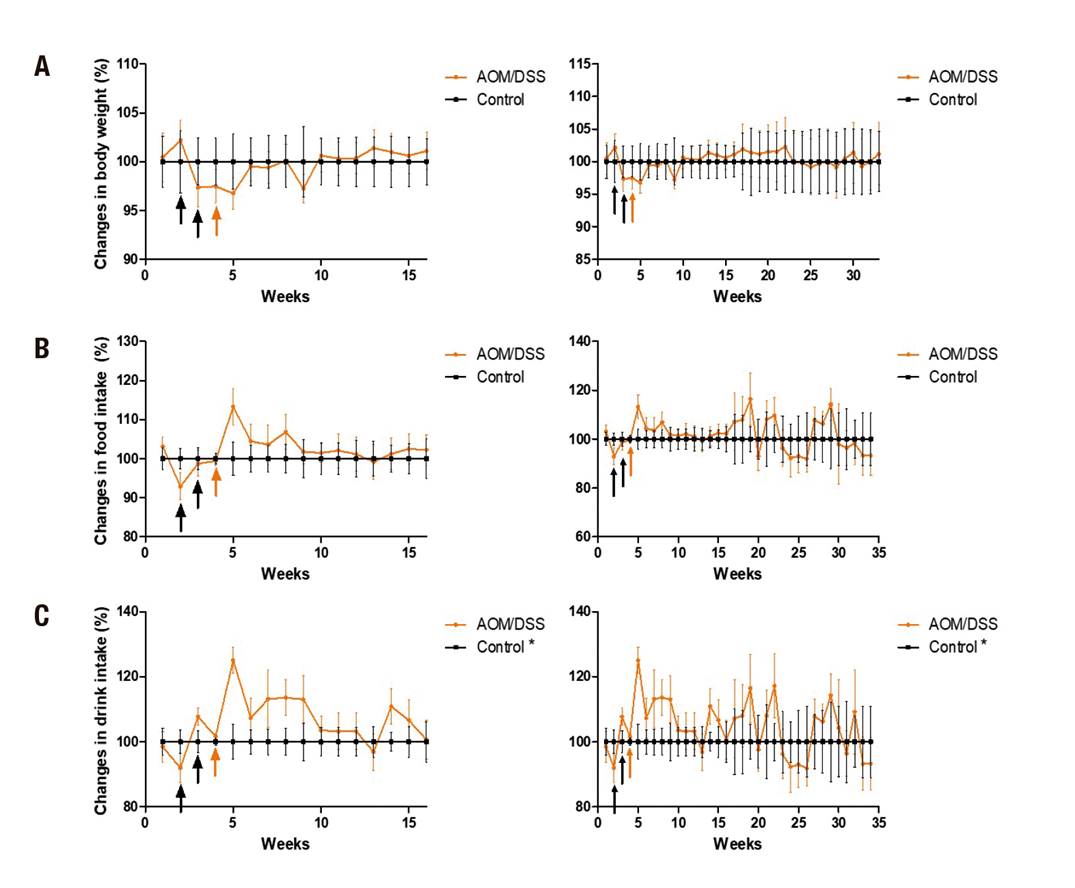
Figure 2. Gain of corporal weight and consumption of food and liquids. A. Gain of body weight. B. Food consumption. C. Fluid intake, at 16 weeks (left) and 32 weeks (right). The data is shown corrected to the control group. The administration of AOM (black arrows) and DSS (orange arrows) is shown. Mean ± SD is shown. Student's t test for independent samples. *p < 0.05
Regarding the weight of sacrificial organs, changes were observed specifically in the chronic stage in the liver weight of the AOM/DSS group and the colon was heavier and longer (p < 0.05) (Table I).
Table I. Comparison of the weight of organs in arbitrary units adjusted at the end of the study

Arbitrary units are shown taking as reference the control group in relation to the weight of the animal at slaughter. Student's t test for independent samples.
*p < 0.05.
The ACF model was confirmed with the methylene blue stain given by Bird et al. 15,19,20. A difference between the two stages was observed, with an increase in the total number of crypts per colon in the chronic stage (14.6 ± 3.8 vs. 22.6 ± 4.2; p = 0.0033) (Fig. 3).
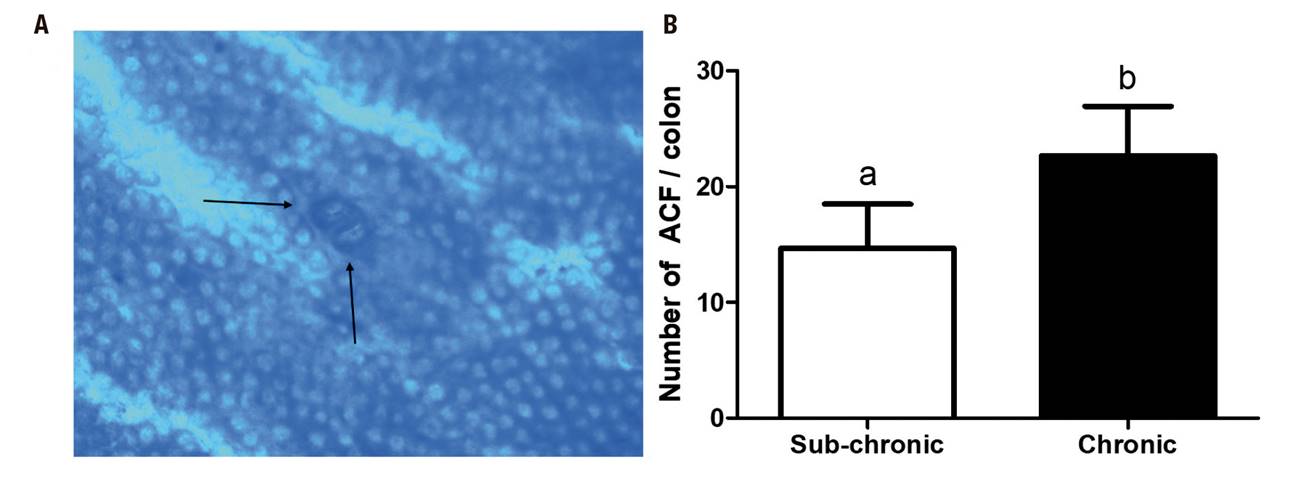
Figure 3. Aberrant crypt foci (ACF). A. Representative micro-image of the colonic mucosa stained with methylene blue (10X). The black arrows represent the location of the ACF. B. Number of ACF/colon. Mean ± SD is shown. Student's t test for independent samples. *p < 0.05
In the blood tests, a decrease in fasting glucose was observed in the AOM/DSS group in both stages (p < 0.05). In the sub-chronic stage only, along with the change in glucose there were statistical changes: total cholesterol decreased and the cells of the white series, lymphocytes and leukocytes increased (p < 0.05).
In histopathological analysis, there were significant histological alterations of the colonic mucosa in the AOM/DSS group in both stages, including infiltration of inflammatory cells into the lamina propria and loss of crypts architecture. We also observed changes in the liver parenchyma in all AOM/DSS-treated animals: a toxic effect involving fibrosis in the hepatic triad, and hyperplasia of the bile ducts. The portal tracts contained small infiltrates of normal-appearing lymphocytes, and there was congestion. Hyperactivity of hepatic regeneration and development of different stages of cystic dilation of the intra-hepatic bile ducts and inflammation and congestion were observed. Lipid vacuoles associated with toxic liver damage were observed. Also we observed renal lesions in the AOM/DSS-treated animals--inflammation and congestion in the glomeruli, reduction of Bowman's chamber, toxic damage that induced glomerulonephritis (Fig. 4).
DISCUSSION
The AOM/DSS study model was designed to generate preneoplastic lesions. Existing literature normally refers to use AOM as a carcinogen inducer, with a minimum dose of 15 mg/kg of weight in different strains of rats 3with a disorder of cell replication. The large majority of colorectal malignancies develop from an adenomatous polyp (adenoma. In the present study, a dose of 10 mg/kg was used in the Sprague Dawley strain. This strain does not generate spontaneous tumors and has the lowest body weight gain among the strains common in this model, for example, Fisher 322 21. This model has the advantage of using less of the AOM reagent, in addition to others, such as the amount of blood and tissues for analysis that can be obtained, ease of manipulation and the high survival rate, in contrast to another alternative, the mouse.
Regarding weight gain, a tendency was observed (not statistically different) at the time of administration of carcinogenic and pro-inflammatory agents. This in contrast to Moreno-Celis (2017) 18, who worked with male Sprague Dawley rats which were administered two injections of AOM (10 mg/kg) and DSS for a week in drinking water, and reported a loss of up to 25% in body weight. But, as in the present study, they also reported a recovery up to 5% at the end of the treatment (Fig. 2). Again in contrast to the present study, they reported a statistical decrease in food consumption. No reports of fluid consumption were found for the present experimental model. In the present study, statistical differences were observed in a higher consumption of liquids in the AOM/DSS group. This could be because certain drugs like AOM produce dry mouth (xerostomia) that may play a role in stimulating thirst. Antipsychotic, antidepressant, and anxiolytic agents are well-known causes of xerostomy 22.
When analyzing the weight of organs at sacrifice, a decrease in liver weight was observed in the AOM/DSS group versus the control group. This probably due to the generation of steatosis reported by several authors. Burlamaqui et al. (2013) 23 studied the hepatic repercussion of colorectal carcinogenesis induced by azoximentane and reported a change in adipose tissue in the liver. They observed that the liver of senile animals can present with degeneration, with areas of fatty, lighter, vacuolated and ballooned cells, with bulkier and irregular nuclei. On the other hand, the colon, as has already been reported 24 well-appreciated, and widely used model of experimental colon carcinogenesis. It has many morphological as well as molecular similarities to human sporadic colorectal cancer (CC, tends to generate hyperplasia and hypertrophy within the crypts, transforming them into ACF, thus increasing the size of the colon in the group treated with AOM/DSS. There are no reports of organ weight at sacrifice in other studies against which to compare our results. Because of that, it is very interesting to observe how they behaved in the AOM/DSS group.
For the induction of ACF, Caderni et al. (1999) 21 used a single dose of 10 mg/kg, among other doses, in Fischer 344 rats, which were sacrificed at 12 weeks after induction of fasting or postprandial AOM. They reported a count of 7.0 ACF/rat, in contrast to the present study, without the administration of DSS, with 2 applications of AOM in Sprague Dawley rats. In the present study more than double the ACF were found, 14.6 ACF/rat. In the present model, only one AOM application is used, reducing the cost of this agent by half. To date, the other studies have been in mice, or with doses greater than 10 mg/kg of weight.
Regarding the hematological analyzes, those of the present study contrast with that reported by Kim et al. (2011) 25. They were carried out in male ICR mice with 3 AOM applications of 10 mg/kg and a week of 2% DSS. They did not report significant changes in the values of white blood cells, red blood cells, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration after the application of AOM. Matkowsky et al. (1999) 26 reported that blood glucose decreases with a single dose of AOM of 100 μg/g in male C57BL/6J mice and in Sprague Dawley rats. Using Crj: CD-1 (ICR) mice with 4 AOM administrations of 15 mg/kg, Hata et al. (2012) did not report a statistical difference between AOM and the control group, but did report a tendency toward increased glucose values with the treatment of AOM 27. This contrasts with Hirose et al. (2003) 28 who reported statistically different blood glucose levels in C57BL/KsJ-db/db mice between the control and the AOM group (15 mg/kg body wt once weekly for 5 weeks), suggesting the possibility of insulin resistance. There is accumulated evidence suggesting that hyperinsulinemia is involved in colon carcinogenesis, as well as in obesity and diabetes. Several epidemiological studies indicate that diabetic patients with hyperinsulinemia have an increased risk of colon cancer 28.
Within the histopathological analyses of target organs of metabolism and AOM excretion, like Kobaek et al. (2004) working with female and male rats of the BDIX/OrlIco strain with a weekly administration of AOM (15 mg/kg) for a period of 2 × 2 weeks separated by a one-week break, we observed a greater number of infiltrating lymphocytes and dilated blood vessels in rats treated with AOM compared to untreated rats. The dilated blood vessels could be a pre-state of hemangiomal tumors, as occurred in the study by Kobaek et al. in the liver of rats treated with AOM after longer latency periods 9. The bile duct hyperplasias could be due to a toxic effect and subsequent regeneration of the liver cells, since pleomorphism is a common finding in this condition. Alternatively, proliferation could also be a direct effect of the carcinogen. As for the kidney, the same thing happens; there are several studies that discuss kidney cancer mediated by AOM 29. But in our study we did not observe any neoplasm, only inflammation in the glomeruli that could be a pre-state of tumoromygenesis in the kidney mediated by AOM. Regarding the colon, aberrant crypt foci were observed in the present study that increased over time in the two study stages, without finding tumors. This being the case, it is not necessary to carry the study model to 32 weeks; 16 weeks is sufficient to evaluate a possible preventive effect against the observed ACF.
CONCLUSIONS
The present experimental study resulted in a highly reproducible incidence of ACF induction at 16 and 32 weeks in a Sprague Dawley rat model. As a study model for the prevention of ACF, it can be carried out for 16 weeks, which makes it a relatively inexpensive model. Likewise, the effect on nutritional markers was determined, and biochemical variations observed, which is important to take into account when reproducing the model to analyze possible preventive agents, in order to avoid false positives. At the same time, side effects associated with the treatment of AOM were observed in organs responsible for its metabolism and excretion. Further studies are needed to determine the mechanism of the changes observed in the present model.

















