INTRODUCTION
In 2019, 463 million adults were living with type-2 diabetes mellitus (T2DM) worldwide (1). In Mexico the prevalence of diabetes in the adult population increased from 7 % in 2006 to 9.4 % in 2016 (2).
Promotion of a lifestyle that includes a heathy diet is essential for the self-management of T2DM in order to control blood glucose and lipid levels, body weight, and blood pressure, and ultimately to reduce the risk of complications related to the disease (3,4). Although it is well known that diet has a pivotal role in the prevention and management of diabetes, there is still a need to better define the optimal dietary approach to improve glycemic control and to manage complications in diabetes (5,6). The effect of the diet on the prevention and management of diabetes has been traditionally based on the intake of single nutrients and individual foods (7). Nevertheless, due to differences in dietary habits and customs among populations, there is growing awareness about the importance of looking at the effects of diet as a combination of foods rather than isolated nutrients or foods (8). Also, dietary patterns such as Mediterranean diet improves glycemic control and other metabolic indicators, compared to low-carbohydrate diet and low-fat diet exclusively (9,10).
Thus, the study of dietary patterns has emerged as an alternative or complementary approach in the prevention and attention to diabetes (11,12). The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) have integrated this approach into their recent dietary guidelines for the management of hyperglycemia in diabetes (13).
In Mexico, the benefit of a healthy dietary pattern (DP) on glycemic control was recently reported, compared to the western pattern and the sweet and dairy pattern in type-2 diabetes (14). Although the diet of Mexicans is characterized by a diversity of traditional and prepared foods, a reduction in the consumption of cereals and legumes has been reported from 1961 to 2013, with an increase in foods with higher energy density (15). Thus, the aim of this study was to identify the dietary patterns associated with metabolic risk factors in a sample of patients with type-2 diabetes who attended primary care clinics.
MATERIAL AND METHODS
STUDY DESIGN AND POPULATION
This is a cross-sectional analysis study in patients with type-2 diabetes seen at primary care clinics of the Mexican Social Security Institute (IMSS, by its acronym in Spanish: Instituto Mexicano del Seguro Social) in the period from January 2017 to October 2018. This study was approved by the National Committee for Ethics and Research of the IMSS. An Ethics and Research Committee approved the protocol. Patients were invited to participate at their local clinics in Mexico City, Mexico. Once their doubts about participating in the study were cleared, the patients accepted to participate by signing an informed consent form. A sample size calculation was performed for the mean difference with the outcome variable of A1c, expecting a mean difference of 0.6 to be detected between groups with healthy versus unhealthy dietary patterns, with a standard deviation of 2.1, a confidence level of 95 %, and a power of 80 %, and a total of 388 patients was obtained.
ELIGIBILITY CRITERIA OF PARTICIPANTS
Patients with less than a 20-year history of type-2 diabetes, ≤ 70 years of age, able to read and write in Spanish, and with A1c > 6.5 % and ≤ 13 % were included. Pregnant patients and those with advanced peripheral neuropathy, retinopathy, or kidney disease were excluded. Clinical assessments and collection of blood samples and all data related to this study were conducted at the Clinical Epidemiology Research Unit of the IMSS Regional Hospital No. 1.
SOCIODEMOGRAPHIC AND MEDICAL HISTORY MEASUREMENTS
Sociodemographic and clinical data were obtained, and a physical examination was performed. Hypertension was defined in those who were previously diagnosed by their physician, confirmed by a review of the patient's clinical record. Systolic (SBP) and diastolic blood pressure (DBP) were measured twice in the left arm after the patient remaining in a sitting position for at least 5 minutes before the first evaluation, and with a 4-min interval between measurements. The mean value of two measurements was used for the analysis. Physical activity was considered to be present when a patient reported having at least 150 min/week of moderate-intensity aerobic physical activity for at least 3 days/week (16).
BIOCHEMICAL MEASUREMENTS
After a 12-h fasting period, venous blood samples were obtained from each patient. Hemoglobin A1c (A1c) levels were measured using the high-performance liquid chromatography method. Fasting glucose, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglyceride serum levels were also obtained by the automated photometric method (Roche Cobas 800 c701).
ANTHROPOMETRY MEASUREMENTS
Weight, height, and waist circumference (WC) were assessed by 2 trained personnel according to the Habicht method and the procedures described by Lohan et al. (17,18). Weight and height were measured using a TANITA™ scale (model TBF-215). WC was measured three times and the mean of the last two measurements was used for the analysis. Body mass index (BMI) was considered to be abnormally high when the value was ≥ 25 kg/m2 (19).
INDICATORS IN RISK LEVELS
Metabolic control at risk was considered when any of the following conditions were met: A1c ≥ 7 %, glucose ≥ 130 mg/dL, TC ≥ 200 mg/dL, HDL-c < 40 in males or < 50 mg/dL in females, LDL-c ≥ 100 mg/dL, and triglycerides ≥ 150 mg/dL (20,21).
Waist circumference was classified at risk when it was ≥ 88 cm in women or ≥ 94 cm in men, adjusting the cutoff point for a Hispanic population (22). For the purpose of this study, uncontrolled hypertension was considered when SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg (21).
DIETARY INTAKE MEASUREMENTS
Dietary intake was assessed using a semi-quantitative food frequency questionnaire (FFQ) validated for the Mexican popula- tion (23). This FFQ assesses the consumption of a standardized portion of 116 foods and beverages (e.g., 1 cup of milk, 1 piece of orange, 1 piece of egg, etc.). Frequency was described as never or consumption on a monthly, weekly, or daily basis over the last year. Included on the list were dairy, eggs, meat and cold cuts, fruit, vegetables, legumes and cereals, Mexican dishes, sweets, drinks (including alcohol), and fats to prepare or dress foodstuffs.
For the purpose of this analysis, we estimated the daily grams consumed of each food item by multiplying each estimated portion of each food item by a factor corresponding to the frequency of intake. Energy, macronutrient, and fiber intake was estimated by using the software program Evaluation System of Nutritional Habits and Nutrient Consumption (Sistema de Evaluaciòn de Hàbitos Nutricionales y Consumo de Nutrimentos), developed by the National Institute of Public Health of Mexico, which contains Mexican food composition data (24).
IDENTIFICATION OF DIETARY PATTERNS
To identify DPs we re-classified the 116 food items into the following groups based on their nutrient composition as shown in the annex 1: 1) full-fat dairy; 2) fruits; 3) vegetables; 4) whole-grain cereals; 5) vegetables oils; 6) legumes; 7) fish, eggs and poultry; 8) red meat; 9) processed meat; 10) refined cereals; 11) sweetened beverages; 12) sugars; 13) fats; 14) traditional foods; 15) fried snacks; and 16) alcohol.
DPs were computed by K-means cluster analysis based on the percentage contribution to total daily intake, in grams, of each food group (25). We examined 2 to 5 solutions to evaluate the best set of clusters to characterize DPs. We identified three clusters that better defined DPs in this sample: 1) fruits and vegetables, 2) full-fat dairy products and sweetened beverages, and 3) diverse with alcohol.
STATISTICAL ANALYSIS
Descriptive statistics of continuous variables are presented as mean ± standard deviation for normally distributed variables, or median (25th, 75th percentile) for those non-normally distributed. Categorical variables are presented as percentages.
For the comparison of continuous variables among clusters we used the one-way analysis of variance (ANOVA) for normally distributed variables, and the Kruskal-Wallis test for the non-normally distributed ones. We also conducted a post-hoc analysis by the Bonferroni method for multiple comparisons among clusters. The Chi-square test was used for comparison of categorical variables.
Simple and multiple linear regression analyses were performed to test the linear association between clusters and cardiometabolic risk factor. The regression coefficient (β) and 95 % confidence intervals (95 % CI) were estimated for all cardiometabolic risk factors, considering the “fruit and vegetable” DP as the reference group. All multivariable linear models were adjusted by sex, age, energy intake, physical activity, and years with diabetes. Linear models for SBP and DBP were further adjusted by BMI, alcohol consumption, and tobacco consumption; glucose and A1c by BMI and hypoglycemic medications; total cholesterol, HDL-c, and LDL-c by BMI; and triglycerides by BMI and alcohol consumption. All covariates were selected based on their potential effect on the studied outcomes.
Simple and multiple logistic regression analyses were performed to estimate the unadjusted and adjusted odds ratios (OR) and 95 % CIs of having a BMI ≥ 25 kg/m2; WC ≥ 88 cm for females or ≥ 94 cm for males; SBP ≥ 140 mg/dL, DBP ≥ 90 mmHg; glucose ≥ 130 mg/dL; A1c ≥ 7 %; TC ≥ 200 mg/dL; HDL-c < 40 mg/dL in males or < 50 in females; LDL-c ≥ 100 mg/dL, and triglycerides ≥ 150 mg/dL. We considered the “fruit and vegetable” DP as the reference group. Multivariate logistic models were adjusted for the same co-variables listed for the linear models. All statistical analyses and data processing were performed using STATA, version 14 (Stata Corp., College Station, TX, USA) for Mac.
RESULTS
Overall, 395 participants were included in this secondary analysis, of which 279 (68.4 %) were female; mean age was 54.6 ± 8.5 years, and the median (25th, 75th percentiles) time of diabetes diagnosis was 6 (3, 11) years.
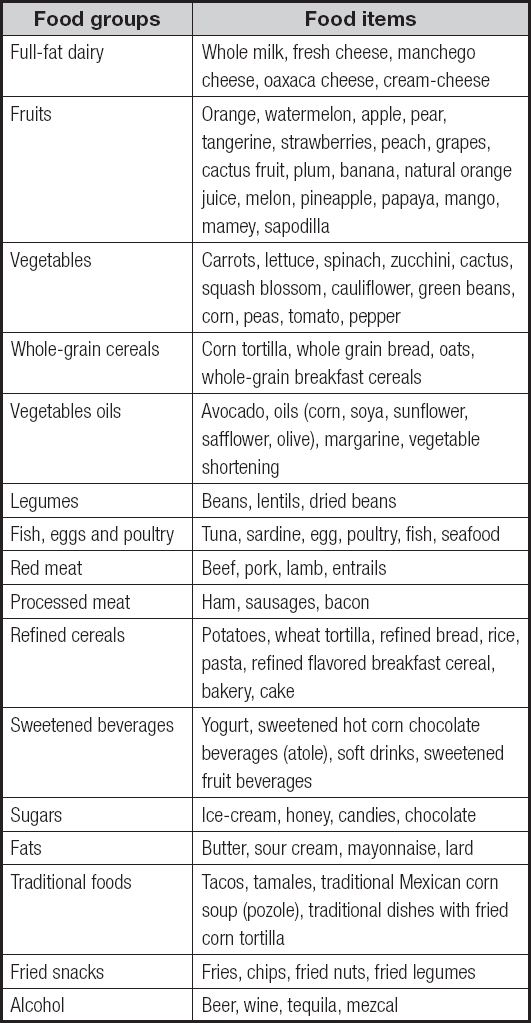
The sociodemographic characteristics of the studied sample by DP are shown in table I. The ‘diverse with alcohol' DP had the highest proportion of males, whilst patients in the ‘fruits and vegetables' DP had the longest duration of diabetes, and the highest proportion of patients with regular physical activity (p < 0.001); similarly, the 'fruits and vegetables' dietary pattern had a lower intake of energy, proteins, carbohydrates, and total and saturated fat (p < 0.001).
Table I. Sociodemographic and clinical characteristics of patients with diabetes (n = 395)
a,bPost-hoc test shows the difference between groups, (significance level of p < 0.05). p-value for comparison between clusters by one-way analysis of variance for normal distributed variables (data are presented as mean ± standard deviation and percentages for categorical variables) or Kruskal-Wallis test for non-normal distributed variables (years of diabetes diagnosis are presented as median (percentiles 25-75) due to lack of normal distribution). Chi-squared test for categorical variables. Means with different superscript are significantly different at a significance level of p < 0.05. Bonferroni post-hoc test.
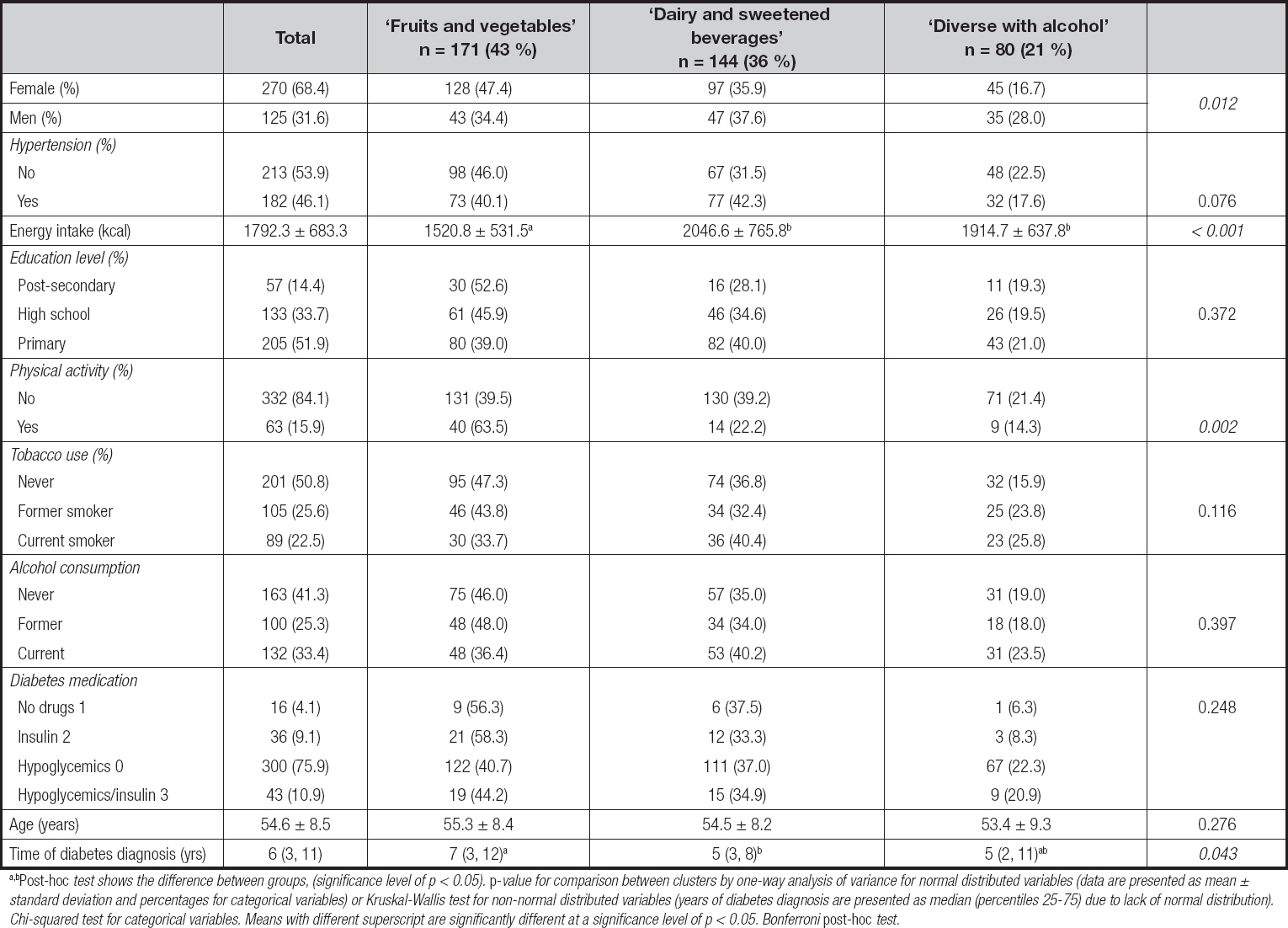
The percentage contribution to total daily intake (in grams) of each food group that characterized each cluster are shown in table II. The first cluster (n = 171), ‘fruit and vegetables', was characterized by patients having the highest consumption of fruits, vegetables, whole-grain cereals, fish, eggs, and poultry. This dietary pattern also showed the lowest intake of refined cereals, sweetened beverages, and traditional foods compared to the other two clusters.
Table II. Percentage of contribution in the diet, with daily intake in grams, of each food group per cluster (n = 395)
A darker highlight denotes the highest percentage of consumption in grams for that cluster compared with the other two. A lighter highlight denotes the lowest percentage of consumption in grams for that cluster compared to the other two.
The second cluster (n = 144), ‘dairy and sweetened beverages', exhibited the lowest intake of fruits, vegetables, whole-grain cereals, vegetable oil, legumes, fish, egg, poultry, processed meat, and alcohol when compared to the other two clusters. The third cluster (n = 80), ‘diverse with alcohol', showed the highest intake of vegetable oil, legumes, red and processed meat, refined cereals, sugar, fats, traditional foods and fried snacks, and alcohol.
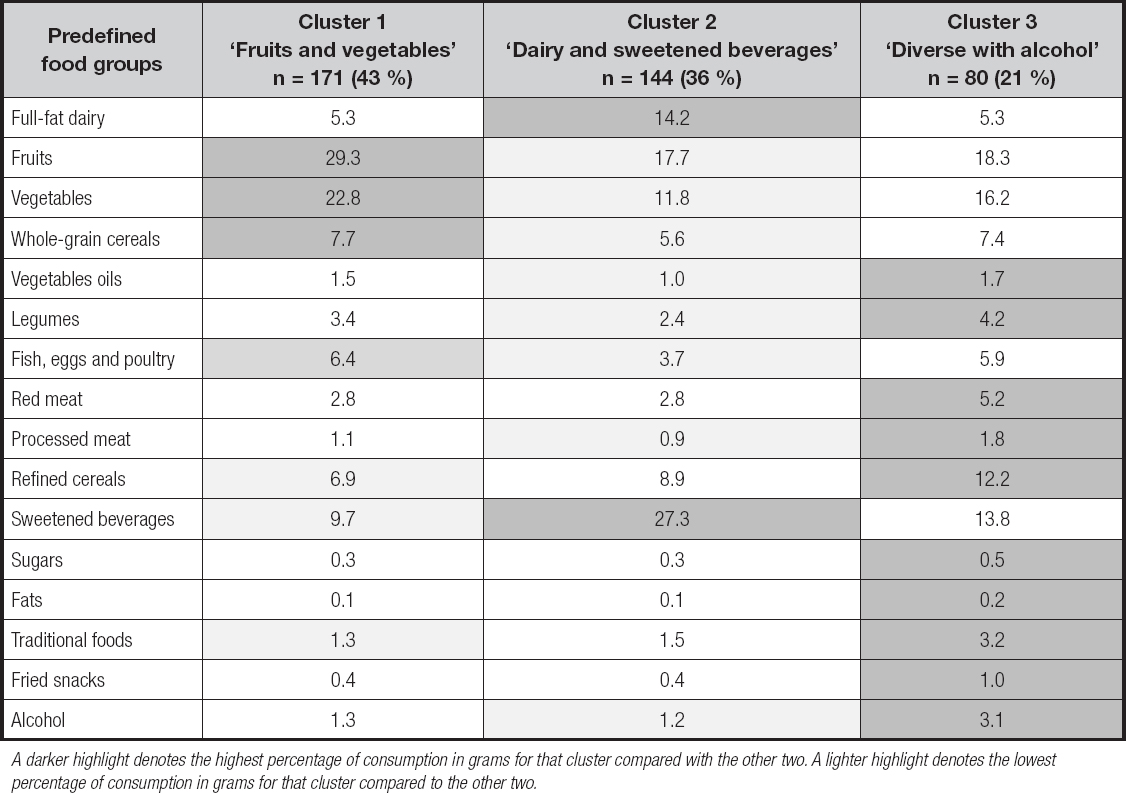
The three clusters that better defined DPs, with anthropometric, clinical and biochemical characteristics, are shown in table III. Differences were found in A1c (p = 0.062) and glucose levels (0.074), which showed a trend to be higher among the patients in ‘dairy and sweetened beverages', as compared to the other two DPs (p = 0.074).
Table III. Anthropometric, clinical and biochemical characteristics of the studied sample by dietary pattern (n = 395)
*Median and percentiles 25-75. BMI: body mass index; A1c: glycated hemoglobin; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol. Data are presented as mean ± standard deviation. p-value for comparison between clusters by one-way-analysis of variance for normal distributed variables or Kruskal-Wallis test for non-normal distributed variables.
A trend towards an unadjusted positive association between the “dairy and sweetened beverages” DP and glucose levels (β = 15.99; 95 % CI: -0.06, 32.03, p = 0.051), as compared to the reference category ‘fruits and vegetables' DP, is shown in table IV. The unadjusted association (β = 0.54; 95 % CI: 0.04, 1.04; p = 0.033) for this DP and A1c levels was also observed after adjusting for co-variates (β = 0.61; 95 % CI: 0.09, 1.12; p = 0.021).
Table IV. Unadjusted and adjusted linear regression analysis for the association between dietary patterns with anthropometric and clinical variables (n = 395)

BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; A1c: glycated hemoglobin; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol. aAdjusted model for sex, age, energy intake, physical activity and years of diabetes diagnosis. bAdjusted model for sex, age, energy intake, BMI, alcohol consumption, tobacco use, physical activity and years of diabetes diagnosis. cAdjusted model for sex, age, energy intake, BMI, hypoglycemic medication, physical activity and years of diabetes diagnosis. dAdjusted model for sex, age, energy intake, BMI, physical activity and years of diabetes diagnosis. eAdjusted model for sex, age, energy intake, BMI, alcohol consumption, physical activity and years of diabetes diagnosis.
Table V. Logistic regression analysis for the association between dietary patterns and metabolic variables (n = 395)
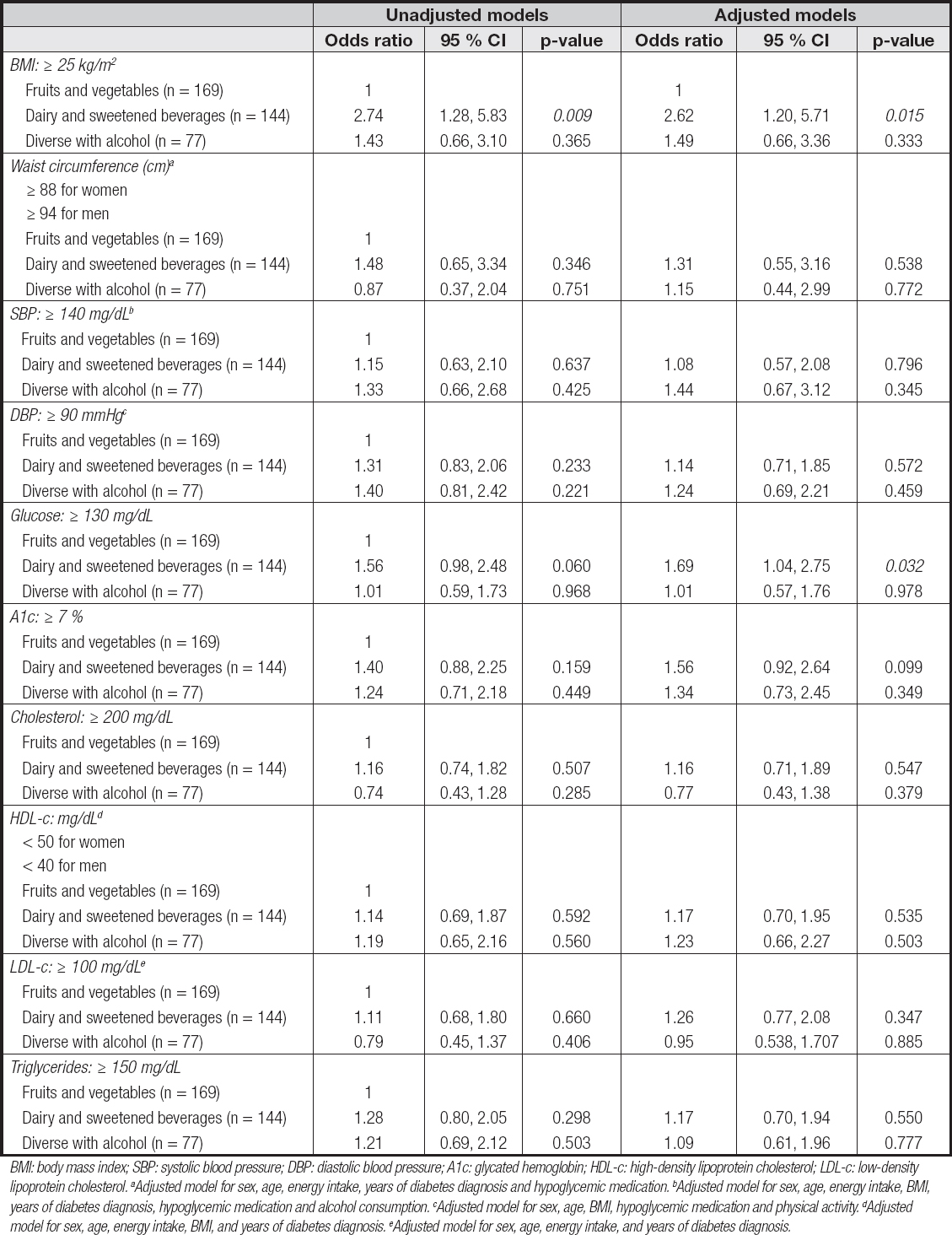
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; A1c: glycated hemoglobin; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol. aAdjusted model for sex, age, energy intake, years of diabetes diagnosis and hypoglycemic medication. bAdjusted model for sex, age, energy intake, BMI, years of diabetes diagnosis, hypoglycemic medication and alcohol consumption. cAdjusted model for sex, age, BMI, hypoglycemic medication and physical activity. dAdjusted model for sex, age, energy intake, BMI, and years of diabetes diagnosis. eAdjusted model for sex, age, energy intake, and years of diabetes diagnosis.
There was also an association between the “dairy and sweetened beverages” DP with BMI ≥ 25 kg/m2 (OR: 2.74, 95 % CI: 1.28, 5.83; p = 0.009). In the logistic model, after adjusting for sex, age, energy intake, hypoglycemic medication, physical activity, and years of diabetes diagnosis, there was a 2.62-fold increased risk of having a BMI ≥ 25 kg/m2 (OR: 2.62, 95 % CI 1.28, 5.71; p = 0.015).
There was also an association of this dietary pattern with a glucose value > 130 mg/dL, (OR: 1.69, 95 % CI: 1.04, 2.75; p = 0.032).
DISCUSSION
The study of dietary patterns is important to describe and combine different food groups of the usual diet, as well as to identify the DP that is associated with poor glycemic control or obesity, among other risk factors for vascular complications, to implement early interventions focused on improving the dietary habits of patients with diabetes.
In this study of an adult Mexican population with type-2 diabetes, a DP characterized by a high intake of full-fat dairy and sweetened beverages was positively associated with A1c levels and an increased risk of having glucose ≥ 130 mg/dL and BMI ≥ 25 kg/m2.
A study in Mexican adults found that participants in the highest tertile of the western DP (characterized by high consumption of soft drinks, refined grains, corn tortillas, and pastries, and low consumption of dairy products, seafood, and whole-grain cereals) had an increased risk for having high fasting glucose and low serum HDL-c levels, in comparison to those in the lowest tertile of this DP (26). More recently, a study in adult Mexican females with excess weight reported that a DP with high consumption of corn tortillas, meats, and legumes was associated with a lower risk of hyperglycemia (27).
Although low-carbohydrate diets high in monounsaturated fatty acids have been widely suggested to improve A1c, it is now recognized that the macronutrient distribution of a diet should be based on an individualized assessment of current eating patterns, preferences, and metabolic goals (28,29). It has also been suggested that there are a variety of eating patterns acceptable for the management of diabetes, such as the Mediterranean-style, low-carbohydrate, and vegetarian or plant-based eating patterns (5,28). In our study, the ‘dairy and sweetened beverages' dietary pattern, which was positively associated with high A1c levels, showed that it had the highest intake of carbohydrates among all DPs. Comparable data were reported with the consumption of soft drinks and fast food > 1 time/week, and the risk of T2DM in the United Arab Emirates (30).
Recent systematic reviews on the effect of different DPs on glycemic control in adults with T2DM had shown that vegetarian, vegan, Mediterranean, and Dietary Approaches to Stop Hypertension DPs reduce A1c by 0.8 % on average, and that the Mediterranean-style diet has the highest reduction in body weight, A1c levels, and delayed diabetes medication requirement, as compared to a low-fat diet (5,31). In our study, higher glucose and A1c levels and glucose at risk (> 130 mg/dL) were observed in the group of ‘dairy and sweetened beverages' DP compared with the other two DPs. Similar results were recently reported in a sample of Mexican patients with type-2 diabetes, where a healthy diet (comparable to the group of ‘fruits and vegetables' in our study) was associated with glycemic control, whereas the western-style or sweets and dairy patterns promoted a poor metabolic control (14).
A network meta-analysis on the comparative efficacy of different dietary approaches on glycemic control in patients with type-2 diabetes concluded that the Mediterranean diet is the most effective dietary approach to improve glycemic control. Even though we find no important differences in lipid profile with any of the three DPs, a cardiovascular benefit of adhering to DPs such as Mediterranean, DASH, Portfolio, Nordic, and liquid food in patients with diabetes has been identified (32,33).
The ADA recommends that a Mediterranean-style eating pattern rich in monounsaturated and polyunsaturated fatty acids may be considered to improve glucose metabolism and to lower cardiovascular disease risk (28). In our study, the ‘diverse and alcohol' DP, which exhibited the higher consumption of vegetable oils and legumes, showed no significant association with metabolic parameters when compared to the ‘fruits and vegetables' one. Of note, the ‘diverse and alcohol' DP also showed the highest consumption of red meat, processed meat, refined cereals, sugar, fried snacks, alcohol and traditional foods, including some prepared with lard, such as tamales, and other dishes based on fried tortilla. In this sense, the type of fats and cooking methods used to prepare some of the traditional Mexican foods may provide no adequate fats. The ‘diverse and alcohol' DP was also characterized by a higher intake of saturated fat compared to the ‘fruits and vegetable' DP. Thus, in order for the Mexican population with diabetes to benefit from a Mediterranean-style diet, there is a need for local dietary guidelines to recommend an increased intake of monounsaturated and polyunsaturated fats while decreasing the consumption of saturated and trans fats and carbohydrates.
Other healthy DPs such as the DASH diet, which is characterized by a high content of fruits, vegetables and whole grains, and a low content of fats, red meat, and sweetened beverages and sweets, similar to that seen in our reference group, should be evaluated in the Mexican population living with T2DM (34).
The relation of a DP with milk fats and sweetened beverages with an increased risk of having a BMI ≥ 25 kg/m2 is consistent with previous reports on the relation of quality of diet with increased risk of diabetes due to weight gain (35,36).
While diet poor quality is related to a lack of glycemic control and persistent obesity in this population, it highlights the importance of considering a personalized diet to improve adherence, considering the particular characteristics of each patient, food culture, social environment, habits, and diabetes treatment goals (37,38).
This study has limitations that should be considered: 1) the study was conducted in patients attending four primary care clinics at the IMSS in Mexico City; thus, these results may not be extrapolated to all patients with type-2 diabetes in Mexico; and 2) the cross-sectional design does not allow causality to be established between the studied DPs and metabolic factors, although the FFQ used in this study to assess dietary intake collects information about food intake over the last year, which makes it possible to assess exposure to food during this period. Finally, it is also important to highlight that this study has the strength of having considered the use of hypoglycemic medication among the adjusting factors, whose effect is critical in the association between dietary intake and metabolic control.
Evaluating DPs is important to characterize the great variety of Mexican foods, which may produce a better prescription of nutritional therapy for patients with diabetes. These patients require planned and personalized educational strategies to promote healthy dietary patterns and reduce the risk of diabetes complications.
In addition to identifying the pattern most commonly associated with poor glycemic control in the Mexican population, it is important to evaluate dietary patterns already widely studied, such as Mediterranean diet and its effect on control indicators in patients with diabetes.
Future studies should confirm the findings of our study and further characterize DPs considering a greater variety of commonly consumed foods in this patient population, which may impact the risk of poor metabolic control.
CONCLUSIONS
In type-2 diabetes patients a dietary pattern characterized by a high intake of full-fat dairy and sweetened beverages was associated with higher A1c levels and increased risk of glucose and BMI among uncontrolled parameters. The dietary pattern of fruits and vegetables offers a protective effect for these indicators of metabolic risk. These Mexican dietary patterns should be evaluated in larger longitudinal studies to identify their effect on the control of glycemia, obesity, hypertension, and dyslipidemia, comorbidities commonly present in patient with diabetes.