INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public health problem in the general population and also in transplant recipients. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) and the KDIGO CKD guidelines define CKD as an estimated glomerular filtration rate (eGFR) of 59 ml per minute per 1.73 m2 of body-surface area or less (< 60 ml/min/1.73 m2), or evidence of kidney damage such as albuminuria or abnormal findings on renal imaging for three months or more 1,2. CKD has a negative impact on the quality of life and is associated with an increased risk of hospitalization and mortality, mainly due to cardiovascular mortality 3.
After solid organ transplantation, a significant decrease in renal function occurs in the majority of patients. Depending on the degree of kidney injury, a large number of patients develop CKD and some develop end-stage renal disease that requires renal replacement therapy 4,5,6,7. The Scientific Registry of Transplant Recipients at the University of Michigan Medical School reported a study, which included 69,321 patients with a median follow-up of 36 months. The incidence of patients with an eGFR < 30 ml/min/1.73 m2 was 8% at 12 months, 13.9% at 36 months and 18.1% at 60 months (5). Of the 11,426 patients with CKD, 28.9% required chronic dialysis or a secondary renal transplantation. O'Riordan et al. reported that 70% of liver transplant recipients had an impaired renal function (eGFR < 60 ml/min/1.73 m2) five years after liver transplantation (LT) 8.
Calcineurin-inhibitor (CNI) therapy has been identified as a major risk factor of CKD in transplant recipients 5,8,9. Several studies have shown that it is possible to reduce the incidence of acute kidney injury (AKI) and CKD by reducing the exposure to CNIs. This is achieved via the combination with non-nephrotoxic immunosuppressive drugs 10,11,12,13, without increasing the risk of graft rejection. Besides, many centers have successfully reduced the exposure to nephrotoxic immunosuppressive drugs in patients with kidney dysfunction. A switch from CNI to an mTOR inhibitor or mycophenolate mofetil (MMF) leads to an improvement of renal function in a variety of patients 14,15.
We hypothesized that the incidence of CKD in the current clinical practice is lower than previously reported, as clinicians are more concerned about CNI induced nephrotoxicity. Therefore, less nephrotoxic immunosuppressive regimens are used. In the present study we aimed to assess the incidence and the evolution over time of CKD in the routine clinical practice in a recent series of Spanish adult liver transplant recipients.
MATERIAL AND METHODS
Study subjects and design
This was an observational, prospective, multicenter study conducted in adult patients (> 18 years) that received a first LT from a brain dead donor. Patients that underwent a transplant from 2009 and 2010, with a GFR ≥ 60 ml/min/1.73 m2 and no evidence of kidney damage at the time of the transplant were included in the study. Post-transplant immunosuppression was modified according to the protocol of each hospital.
The study was performed according to the International Guidelines for the Ethical Review of Epidemiological Studies (Council for the International Organizations of Medical Sciences [CIOMS], Geneva, 2008) and the Declaration of Helsinki (Seoul, October 2008). The study was reviewed and approved by the Clinical Research Ethics Committee of all participating hospitals. All patients gave their written informed consent prior to inclusion in the study.
CKD definition and classification
CKD was defined according to the NKF-K/DOQI and the KDIGO CKD guidelines 1,2. The classification of CKD stages was as follows:
Stage 1: normal or increased GFR (> 90 ml/min/1.73 m2).
Stage 2: mild reduction in GFR (60-89 ml/min/1.73 m2).
Stage 3: moderate reduction in GFR (30-59 ml/min/1.73 m2).
Stage 4: severe reduction in GFR (15-29 ml/min/1.73 m2).
Stage 5: kidney failure (GFR < 15 ml/min/1.73 m2 or dialysis).
GFR was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation 16. The progression of CKD was defined as a change from stages 1, 2 or 3 to stage 4 and from any prior stage to stage 5.
Modifications of the CNI dose and levels and the use of alternate immunosuppressive regimens in patients with kidney dysfunction were defined according to the center protocol. Any therapy that slows the rate of progression in patients with CKD, independent of any modification of the immunosuppressive regimen, was administered according to the center protocol.
Data collection
The study was conducted according to the usual clinical practice management. Demographic and general data of recipients and donors were recorded. This included the underlying liver disease, the presence of other comorbidity, date of LT, use of medication and laboratory data at the time of transplantation.
Patients were assessed at six months post-LT (baseline) and at six-month intervals for two years; the final visit was performed 30 months after the LT. The following laboratory data were documented: glycemia and glycated hemoglobin, lipid profile, renal function (serum creatinine [sCr] and eGFR), hematology and biochemical data and the dose and level of immunosuppressive drugs. The following variables were also collected every six months up to month 30 post-LT: weight, body mass index, blood pressure, concomitant treatment (antihypertensive, antidiabetic and hypolipidemic drugs), graft rejection episodes and infection episodes.
Statistical analysis
The sample size calculation was based on the published proportion of stage 4 CKD three years after LT (13.9%) 5, the capacity to detect a difference of at least 7% in this proportion, an alpha error of 0.05, a beta error of 0.20 and a 3% estimate of missing patients. The sample size should be 219 patients according to these assumptions. Descriptive statistics were generated for the assessed variables, including the total number of valid values. Continuous variables were described by measures of central tendency and dispersion and absolute and relative frequencies were used to describe categorical variables. The 95% confidence interval for the main variable was calculated. A comparison of two continuous variables was performed using the Mann-Whitney test (for non-pair-wise data) or Wilcoxon test (for pair-wise data) for parametric variables. The Chi-squared test (or the Fisher exact test, when required) was used to compare proportions and frequency distributions. The SPSS Version 13.0 software was used and all analyses were bilateral with a significance level 0.05.
RESULTS
Two hundred and thirty LT recipients (74.3% men) with mean age 55.7 ± 9.0 years were enrolled in the study. The sociodemographic and clinical characteristics of the patients are shown in Table 1. Mean time from LT to study inclusion was 6.4 months and the most frequent indications for transplantation were cirrhosis (56.1%) and liver cancer (35.2%). Four patients required transient hemodialysis within the first month after transplantation.
Table 1 Sociodemographic and baseline clinical characteristics of patients (n = 230)
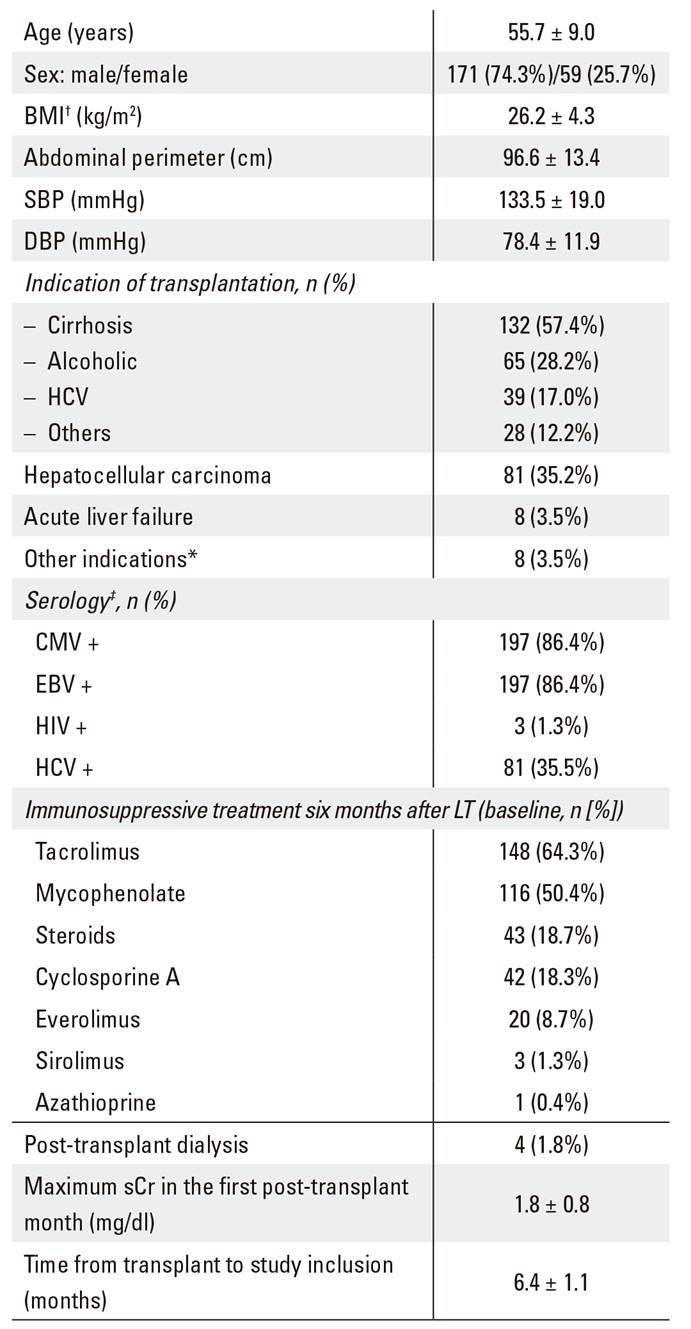
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; ADA: American Diabetes Association; sCr: serum creatinine. Data expressed as mean ± SD for continuous and as n (%) for categorical variables. *Other indications: familial amyloidotic polineuropathy (three patients), liver metastases of a neuroendocrine malignancy (two patients), polycystic liver disease (two patients) and Carolí's disease (one patient). †n = 216; ‡n = 228; §n = 228; ǁn = 227.
Evolution of kidney function
Kidney function rapidly worsened within the first months after LT and stages 3 and 4 CKD were reported in 31.7 and 0.4% of the patients at six months post-LT (all patients had a GFR ≥ 60 ml/min/1.73 m2 before LT), respectively. However, kidney function improved from that time point and the mean eGFR increased from 72.3 ml/min/1.73 m2 at month 6 post-LT to 76.5 ml/min/1.73 m2 at month 12 and 75.6 ml/min/1.73 m2 at month 30 (p < 0.01) (Fig. 1A). The mean levels of sCr significantly decreased from 1.13 mg/dl at month 6 to 1.07 mg/dl at month 12 and 1.09 mg/dl at month 30 (p < 0.05) (Fig. 1B). One hundred and four (45.2%) patients had sCr values ≥ 1.3 mg/dl at least once during the study. However, the proportion of patients with sCr ≥ 1.3 mg/dl did not change significantly during visits.

Fig. 1 Evolution of the (A) mean glomerular filtration rate (± SD) and (B) mean serum creatinine (± SD) during the study period (6-30 months after transplantation) in 205 patients with values at all the time-points of the study (GFR: glomerular filtration rate; LT: liver transplantation; SD: standard deviation).
CKD was not progressive during the study period. As shown in Figure 2, the percentage of patients with GFR ≥ 60 ml/min/1.73 m2 increased from 67.8% at month 6 post-LT to 68.8%, 73.4%, 70.7% and 73.1% at months 12, 18, 24 and 30 post-LT, respectively. The percentage of patients with stage 3 CKD decreased from 31.7% at month 6 to 26.4% at month 30. The percentage of patients with stage 4 CKD remained unchanged during the study; 0.4% at month 6 versus 0.5% at the end of the study, which was non-significant. None of the patients progressed to stage 5 end-stage kidney disease that required chronic dialysis treatment or a renal transplantation. Renal function was stable in patients that received tacrolimus or cyclosporine as a monotherapy and trended to improve in patients who received other immunosuppressive therapies (Supplementary Fig. 1).

Fig. 2 Patients with a glomerular filtration rate lower than 60 ml/min/1.73 m2, between 60 and 90 ml/min/1.73 m2 and ≥ 90 ml/min/1.73 m2 in 205 patients with values at all the time-points of the study (GFR: glomerular filtration rate; LT: liver transplantation).
Evolution of immunosuppression
Initial postoperative immunosuppression consisted of a CNI based regimen in all patients; 99.6% of the patients remained on CNI at six months post-LT, 67.4% were on an anti-proliferative agent, 36.1% were on corticosteroids and 10.6% were on mTOR inhibitors. Figure 3 shows the evolution of the immunosuppression therapy used during the study. The proportion of patients on CNI monotherapy increased during the study. An increase in the proportion of patients free of CNI was also found. Interestingly, 20% of patients remained on steroids up to 30 months after LT and there was a gradual decrease of tacrolimus levels over time (Fig. 4).
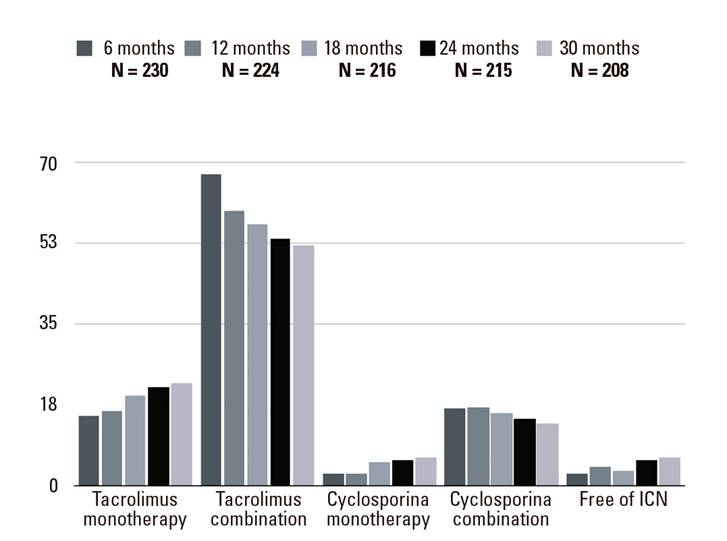
Fig. 3 Evolution of immunosuppressive treatment during the study period (6-30 months after transplantation). Values expressed as percentages.
DISCUSSION
In our case series, a rapid decrease in renal function occurred in a significant proportion of patients within the first months after LT, most probably due to CNI nephrotoxicity. All the patients in this series had a pre-transplant GFR ≥ 60 ml/min/1.73 m2 and approximately one third evolved to stage 3 CKD as early as six months after LT. Previous studies have shown that having a low GFR in the first months post-transplant (one, three and six months post-transplant) is an independent predictor of CKD 17,18,19. Thus, many centers aim for lower target levels of CNI in patients with kidney dysfunction 20.
The proportion of patients with stage 4 CKD (eGFR < 30 ml/min/1.73 m2) in this study remained at 0.4-0.5% up to 30 months post-LT. This rate is similar to the estimated prevalence of stage 4 CKD in the general population (0.4% 21)) and lower than other previously reported rates in liver transplant recipients 5. For example, the cumulative incidence of stage 4 CKD were 8% at 12 months, 13.9% at 36 months and 18.1% at 60 months in the study by Ojo et al. 5. The greater percentage of patients with advanced CKD in this study might be explained by the significant proportion of patients (26.8%) with a pre-transplant GFR < 60 ml/min/1.73 m2, which is a known risk factor for subsequent CKD 19. Furthermore, the higher levels of CNI frequently used at the time of the study.
In our series, CKD was not progressive during the study and seemed to recover over time after LT. In this regard, the percentage of patients with stages 1 and 2 increased over the time and the percentage of patients with stage 3 decreased. As mentioned previously, the percentage of patients with stage 4 remained unchanged and no patients progressed to stage 5. The low risk of developing progressive CKD after LT is probably related to the introduction in recent years of new non-nephrotoxic drugs in the immunosuppressive regimens, such as MMF or mTOR inhibitors. This has allowed reduced doses of cyclosporine A and tacrolimus and led to a reduction of nephrotoxic and cardiovascular-related adverse events 19,22,23,24,25,26,27,28,29. Varo et al. 18 reported that transplanted patients from 1999 onwards had a lower risk of developing CKD than those transplanted prior to 1999, although this effect was attributed to a longer evolution time and consequently, an increased exposure to immunosuppressive treatment and their toxic effects in those patients transplanted before 1999. However, the putative effect of the more recent immunosuppressive regimens cannot be ruled out. In fact, non-nephrotoxic drugs were introduced in some cases after a clinical diagnosis of CKD in that study. The patients transplanted in more recent years have most likely benefited from this treatment. In our study, more than half of the patients received mycophenolic acid and 10-15% mTOR inhibitors. Both types of drugs may have reduced CNIs doses and levels and consequently, have reduced secondary toxic effects in liver transplant patients. In this regard, the GFR and sCr levels improved significantly in our study versus the baseline level. This improvement seemed to be more evident in patients who received non-nephrotoxic drugs. As patients that received CNI monotherapy at the end of follow-up maintained a stable renal function throughout the study, whereas the mean eGFR increased in patients that received other immunosuppressive therapies. The difference between both groups did not reach statistical significance, although this observation reinforces the potential benefit of the use of other drugs to reduce the nephrotoxicity of CNI. In any case, the design of the present study did not allow us to demonstrate that the evolution of renal function is a consequence of reduced CNI doses.
The limitations of the present study are those that are typical of observational studies. These include the non-random assignment and confounding, a relatively short follow-up of 2.5 years, a lack of data for some potential confounders and mainly Caucasian participants. Furthermore, the low number of patients with stage 4 CKD did not allow an analysis of risk factors. However, this study has some attractive features including the relatively large sample size of the cohort, the prospective design and the fact that the patients are representative of the routine clinical practice in Spain. The results of this study should not be generalized to all populations of LT recipients, as the cases included in the present study had a normal pre-transplant renal function.
In summary, up to one third of the patients had a moderate reduction in GFR (30-59 ml/min/1.73 m2) during the first months after LT. However, kidney function tended to stabilize or improve during the year. Furthermore, CKD was a progressive disease to a severe reduction in GFR or kidney failure over time in only a few patients. Our results suggest that in the current clinical practice, de novo post-transplant CKD may be a non-progressive disease.
















