My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Archivos de Zootecnia
On-line version ISSN 1885-4494Print version ISSN 0004-0592
Arch. zootec. vol.63 n.243 Córdoba Sep. 2014
https://dx.doi.org/10.4321/S0004-05922014000300006
Effect of additives on fermentation of cassava leaf silage and ruminal fluid of west african dwarf goats
Efecto de aditivos sobre la fermentación de ensilaje de hojas de yuca y el fluido ruminal en cabras enanas de África Occidental
Oni, A.O.1*; Sowande, O.S.2; Oni, O.O.3; Aderinboye, R.Y.1; Dele, P.A.4; Ojo, V.O. A.4; Arigbede, O.M.4 and Onwuka, C.F.I.1
1Department of Animal Nutrition. Federal University of Agriculture. Abeokuta. Nigeria. *profoni2003@yahoo.com
2Department of Animal Production and Health. Federal University of Agriculture. Abeokuta. Nigeria.
3Department of Agricultural Education. Federal College of Education. Abeokuta. Nigeria.
4Department of Pasture and Range Management. Federal University of Agriculture. Abeokuta. Nigeria.
SUMMARY
A study was conducted to investigate the effects of different additives on the fermentation quality of ensiled cassava leaves and its effects on the ruminal fluid parameters using eighteen West African dwarf goats. Cassava leaves were ensiled alone (ECF), with 5 % (w/w) molasses (ECFM) and caged layer waste (ECFP) respectively as additives for 30 days. Results of chemical composition of the additive and non-additive silages indicated that ensiling with 5 % molasses reduced the DM content from 252.4 g/kg in ECF to 238.9 g/ kg in ECFM and increased with 5 % caged layer waste (ECFP) to 267.6 g/kg. The CP content of ECF was 207.6 g/kg and this decreased to 198.5 g/kg DM in ECFM. Addition of molasses and caged layer waste caused a reduction in the HCN contents from 95.8 mg/kg in non-additive silage (ECF) to 89.3 mg/kg in ECFP and 84.7 mg/kg in ECFM. The mean pH of non-additive silage of 3.66 was significantly (p<0.05) different from the value of 4.29 in ECFP. Animals fed silage ensiled with molasses (ECFM) had a DM intake of 503.42 g/d, followed by values of 485.17 g/d and 458.43 g/d for animals fed ECF and ECFP respectively. Crude protein intake was similar in ECF and ECFM but higher (p<0.05) than ECFP and ranged from 161.28172.71 g/d. The ruminal fluid parameters indicated no significant differences (p>0.05) in the pH, bc and lactic acid concentration in both the non-additive and additive silages. Ammonia-nitrogen concentration (NH3-N) however, ranged significantly (p<0.05) from 15.93 ± 0.20 (mg/dL) in ECF to 22.43 ± 0.61 (mg/dL) in ECFP. The study showed that ensiling cassava leaves with 5 % molasses and caged layer waste improved the silage conditions and the ruminal fluid parameters of West African Dwarf goats.
Key words: Buffering capacity. Caged layer waste. Molasses. Volatile fatty acids. WAD goats.
RESUMEN
Se realizó un estudio para investigar los efectos de diferentes aditivos sobre la calidad de la fermentación de ensilaje de hojas de yuca y sus efectos sobre los parámetros del fluido ruminal en dieciocho cabras Enanas de África occidental. Las hojas de yuca fueron ensiladas, durante 30 días, solas (ECF) y con adición de 5 % de melaza (ECFM) o excretas de ponedoras en batería (ECFP) como aditivos. La adición de 5 % de melaza redujo el contenido de materia seca, 252,4 g/kg en ECF, a 238,9 g/kg en ECFM; con adición de 5 % de excretas (ECFP) la MS aumentó hasta 267,6 g/kg. El nivel de PB (207,6 g/kg en ECF) disminuyó a 198,5 g/kg en ECFM. Tanto la adición de melazas o excretas determinaron la reducción del contenido de HCN desde 98,5 g/kg en ECF (sin aditivos) a 89,3 en ECFP y 84,7 g/kg en ECFM. El pH medio del ensilaje sin aditivos (3,66) fue menor (p<0,05) que el de ECFP (4,29). La ingesta en los animales alimentados con ensilado melazado (ECFM) fue de 503,43 g/d; 485,17 g/d para ECF y 458,43 g/d para ECFP. La ingestión de PB varió entre 161,28 y 172,71 g/d, siendo similar en ECF y ECFM y más baja (p<0,05) en EFCP. Los parámetros del fluido ruminal no mostraron diferencias significativas en pH, capacidad tampón o concentración de ácido láctico para ninguno de los tipos de ensilado. Sin embargo, la concentración de nitrógeno amoniacal (NH3-N) varió (p<0,05) entre 15,93 ± 0,20 (mg/dL) en ECF a 22,43 ± 0,61 (mg/dL) en ECFP. El trabajo demostró que el ensilaje de hojas de yuca con 5 % de melazas o excretas de aves en batería, mejoró su calidad y repercutió favorablemente sobre los parámetros ruminales de cabras Enanas de África Occidental.
Palabras clave: Ácidos grasos volátiles. Cabras WAD. Capacidad tampón. Excretas de ponedoras. Melaza.
Introduction
Recently interest has been focused on foliage from cassava (Manihot esculenta 'Crantz') as a supplemental feed for ruminants. Cassava is known as a highly productive tropical crop that is traditionally cultivated to produce roots for human consumption or for starch production. Ravindran and Rajaguru (1988) reported the yield of cassava leaves to be as much as 4600 kg DM ha-1 when taken as a by-product at root harvesting. Nigeria is the leading producer of cassava. As a crop whose by products have a wide array of uses, cassava is the most important food crop for Nigeria by production quantity next to yam, which is the most important food crop by value (FAOSTAT, 2012). Nigeria is the world's largest producer of cassava with other top producers being Indonesia, Brazil, Thailand, the Democratic Republic of Congo, Ghana and Angola. It has been estimated that in 2010, Nigeria's production of cassava reached 37.5 million tonnes and increased to about 54 million metric tonnes in 2012 (FAOSTAT, 2012). The country has consistently been ranked as the world's largest producer of cassava since 2005 (FAOSTAT, 2012).
The Federal Government of Nigeria's policy on cassava production for both local and international market has adopted a strategy of adding 10 percent cassava flour in wheat flour for local industries from January, 2005. Therefore, a large quantity of cassava leave is generated annually. In dealing with the rainy season crop harvest, and due to the difficulties in hay storage, ensiling is considered to be the preservation technique with the greatest potential for protein rich foliage (Man and Wiktorsson, 2002). Many authors have also reported that ensiling cassava leaves is an appropriate method to conserve it for dry season feeding (Limon Loeza, 1992) as an effective way of reducing cyanide (HCN) content (Tewe, 1991 and Nguyen Thi Loc et al., 1996). However, according to Man and Wiktorsson (2002), ensiling is less advantageous because it increases labour costs and the risks of unfavourable microbial processes during the time of ensiling and storage, which affect the palatability, nutrient content and may lead to the development of toxic substances. Fermentation also contributes to some extent in reducing the deleterious effect of cassava forage. The HCN content was reduced by 68 % after 2 months of ensiling and the palatability increased (Man and Wiktor-sson, 2001; 2002). However, such cassava leaves need good weather for drying and special techniques to limit the loss of dry leaves. Ensiling will be a suitable way of preserving the leaves, but with high content of nitrogen and low content of water soluble carbohydrates (WSC). Therefore, for green fodder materials like cassava leaves, silage additives should be added to ensure successful fermentation (Petterson, 1988). Molasses, a common feed ingredient in the tropics, is commonly used as an additive for ensiling low WSC tropical forages to improve the silage quality. The present study was conducted to investigate the effects of different additives on the fermentation quality of ensiled cassava leaves and ruminal fluid parameters of West African Dwarf goats.
Materials and methods
LOCATION AND CLIMATE OF THE STUDY AREA
The study was conducted in the Small Ruminant Experimental Unit, College of Animal Science and Livestock Production, University of Agriculture, Abeokuta, Nigeria. The experimental site was located in the derived savannah vegetation zone of south-western Nigeria. The climate in this area is tropical, with a wet season from March to October and a dry season from November to February. Annual rainfall averages about 1100 mm and the peak rainfall occurs in the period June-September. The temperatures and relative humidity during the period of study were 32 -35 oC and 75-83 %, respectively.
EXPERIMENTAL ANIMALS, MANAGEMENT AND DIETS
Eighteen West African Dwarf (WAD) male goats aged 12-15months with an average live weight of 7.17-7.23 ± kg were used for the feeding trial. The animals were intensively managed in well-ventilated individual pens (1.2x0.90 m), in an open-sided type of house with corrugated aluminium roofing sheet and a wooden floor, which had been disinfected with Izal solution before the arrival of the animals. The goats were vaccinated against Peste des petit de ruminant (PPR), given prophylactic treatments, which consisted of intramuscular application of oxytetracycline and Vitamin B complex at the dosage of 1mL/10 kg body weight of the animal. They were dewormed with 1 mL/10 kg body weight of albendazole® and treated against ectoparasites with 0.5 mL/10 kg body weight oflvomec®. They were allowed an adaptation period of four weeks during which they were maintained on elephant grass and concentrate supplement with gradual withdrawal of the grass. Fresh water was supplied ad libitum. Cassava foliage (leaves + petiole) were evaluated in ensiling studies with or without additives. Molasses and caged layer waste were used as additives at 5 % (w/w) of fresh material. Cassava leaves of TMS 30572 variety of about 12 months old, were collected in the field immediately after root harvesting and allowed to wilt for a minimum of 12 hours in a well-ventilated shed. Cassava leaves were thoroughly mixed with the additives in different silos, which constituted different treatments with 5 % additive as follows: Treatment 1: cassava leaves alone; Treatment 2: cassava leaves + molasses and Treatment 3: cassava leaves + caged layer waste.
Each treatment was replicated four times using a total of 12 plastic containers with capacity to contain 200 kg fresh materials each. The cassava leaves were chopped into 4 -5 cm length, packed in polyethylene bags and placed into the plastic containers. Molasses and caged layer waste were mixed with the chopped leaves and the materials were manually compacted. After filling, the tops of the bags were bound with rubber band string and pressed by placing about 5 kg stones on top throughout the ensiling period. The containers were stored for a month to allow fermentation. The experimental animals were divided into 6 animals per treatment and offered the different ensiled materials. A basal diet of 1 kg Gmelina arborea foliage was offered daily to each goat while the cassava leaf silage was offered ad libitum to provide dry matter intake on at least 5 % of their body weight. During the 84-days experimental period, quantities of feeds offered and refused were measured daily to compute feed intake on DM basis. During the last two days of each 15 day period, ruminal fluids were collected before feeding in the morning using stomach tubes to determine ammonia, buffering capacity and volatile fatty acids (VFAs).
CHEMICAL ANALYSES OF SILAGE SAMPLES
The silage samples from all the bags were thoroughly mixed and a sub-sample of 1 kg each taken. The sub-sample was adequate to provide sub sub-samples required for chemical analyses, pH determination and dry matter contents. The samples were labelled and kept in a deep freezer at -10 oC for laboratory analysis. Samples of silage were analysed for their proximate contents (AOAC, 2000, reference ID numbers DM 930.15, CP 984.13, Ash 942.05 and EE 963.15). Neutral detergent fibre (NDF) and acid detergent lignin (ADL) were determined by the methods of Van Soest et al. (1991) NDF was analysed using sodium sulphite and amylase and expressed with residual ash. HCN content of the silage samples were determined by the alkaline titration method (AOAC, 2000). Condensed tannin was determined by the butanol-HCl method (Terrill et al., 1992). Volatile fatty acids in the silage samples were determined by the method of Wiseman and Irvin (1957). Ammonia-N in silage samples was determined using the method described by Lanyansunya et al. (2007). The silage pH was determined by weighing 40 g from each treatment and soaked in 200 mL of cool distilled water for 12 hours. The mixture was then filtered and the supernatant divided into 4 aliquots each for pH determination using JENWAY pH meter, model 3150. The ruminal pH was determined immediately after collection using JENWAY pH meter, model 3150. Samples of ruminal fluid were filtered through cheese cloth and pour into centrifuge tubes containing 1 mL of 0.1 N HCl for preservation and determination of ammonia nitrogen (NH3-N) by the standard Kjeldahl procedure (AOAC, 2000), or 1 mL of 25 % metaphosphoric acid for VFA determination using gas liquid chromatography.
All data obtained were subjected to oneway analysis of variance in a Completely Randomized Design. Significantly different (p<0.05) means were compared by Duncan Multiple Range Test using SPSS (1999).
Results
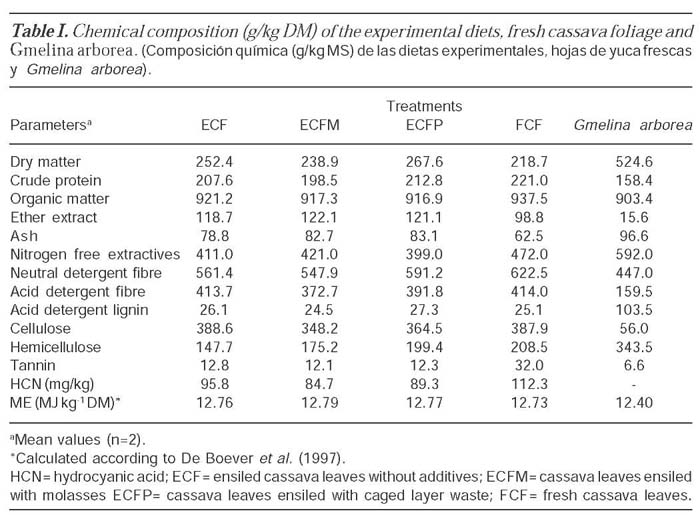
The chemical composition of the experimental diets of ensiled cassava leaves (ECL) with or without additives is shown in table I. The DM in ECF was 252.4 g/kg while ensiling with 5 % molasses (ECFM) reduced the DM to 238.9 g/kg and increased with 5 % caged layer waste (ECFP) to 267.6 g/kg. The CP content of ECF was 207.6 g/kg DM. The crude protein content of ECFM was 198.5 g/ kg DM while a value of 212.8 g/kg DM was recorded in ECFP. There was a reduction in the NDF with addition of molasses while addition of poultry manure increased the NDF compared with ensiling without additive. The addition of molasses and caged layer waste reduced the ADF content from 413.7 g/kg DM in ECF to 372.7 g/kg DM in ECFM and 391.8 g/kg DM in ECFP. The HCN content in the silage was highest (95.8 mg/kg) in ECF.
The results for pH, buffer capacity and fermentation quality of ensiled cassava leaves with or without molasses and caged layer waste are summarized in table II. The mean pH value of the non-additive silage was 3.66 ± 0.06 and was significantly (p<0.05) different from the value of 3.17 ± 0.04 obtained with addition of molasses but not significantly (p>0.05) different from the value of 4.29 ± 0.09 obtained with the addition of poultry manure. The concentration of ammonia nitrogen (NH3-N) was 8.81 ± 0.24 (mg/dL) in ECF and significantly differ (p<0.05) from the value of12.48 ± 0.96 (mg/ dL) obtained in ECFP but similar with the value of 6.89 ± 0.36 (mg/dL) in ECFM.
The mean concentrations of acetic acid were 0.41 ± 0.31 %, 0.58 ± 0.27 % and 0.70 ± 0.29 % for ECF, ECFM and ECFP respectively. Lactic acid concentrations were different among treatments (p<0.05) with mean values of 4.16 ± 0.39 %, 5.13 ± 0.11 % and 6.91 ± 0.09 % in ECF, ECFM and ECFP respectively.
The results of nutrient intake of WAD goats fed cassava leaves with or without molasses and caged layer waste are shown in table III. Dry matter intake in silage significantly (p<0.05) ranged from 203.15 g/ d in ECFP to 246.86 g/d in ECFM. Total DM intake was significantly (p<0.05) highest in ECFM with a value of 503.42 g/d while similar and lowest values were obtained for ECFP and ECF respectively. The CP intake was significantly (p>0.05) similar in all the treatments and ranged from 161.28 g/d in ECFP to 173.08 g/d in ECFM. Values obtained for NDF intake were 325.9 g/d, 347.1 g/d and 364.0 g/d for ECFP, ECF and ECFM respectively.
Table IV shows the effects of ensiled cassava leaves with or without molasses and caged layer waste on rumen fluid parameters of WAD goats. There was no significant difference (p>0.05) in the pH, BC and lactic acid concentration of the ruminal fluid parameters in both the non-additive and additive silage. Ammonia-nitrogen concentration (NH3-N) ranged significantly (p<0.05) from 15.93 ± 0.20 (mg/dL) in ECF to 22.43 ± 0.61 (mg/dL) in ECFP. ECFM had the highest values of 36.83 ± 0.20 % and 11.83 ± 0.29 % for acetic and butyric acids respectively and was significantly (p<0.05) different from the values of 35.27 ± 0.29 % for ECFP acetic acid and 35.80 ± 0.29 % for ECF acetic acid which were not significantly (p>0.05) different from each other. ECFP recorded the lowest butyric acid concentrations.
Discussion
The result of the chemical composition of ensiling cassava leaves with or without additives indicates that the addition of molasses reduced the DM concentration of the ensiled materials while the opposite trend was observed when caged layer waste was added. The DM concentration of 252.4, 238.9 and 267.9 g/kg DM obtained for ECF, ECFM and ECFP respectively were below the values of 329 and 363 g/kg DM obtained by Man and Wiktorsson (2001) for cassava tops silage with 0 and 6 % molasses and value of 258 g/kg DM obtained for cassava tops silage by Man and Wiktorsson (2002). Khang and Wiktorsson (2004) obtained value of 390.1 g/kg DM for ensiled cassava tops while Pinho et al. (2004) reported values of 25 and 27.7 % for wilted and non-wilted aerial parts of cassava plants. According to these authors, wilting the aerial part of the cassava plants resulted in an increase in DM concentration by 10.8 % which was considered low. Nussio (1991) cited minimum DM concentration range of 25 to 27 % for the material to be properly ensiled so that the DM concentration will not interfere with the fermentation process within the silo. With DM contents between 250-350 g/kg DM and pH values below 4.5, most silage samples can be considered to be good (Petterson, 1988). The CP content in the fresh material reduced slightly after ensiling and the reduction was more evident with the addition of molasses and poultry manure. Values obtained are similar to values reported by several authors (Man and Wiktorsson, 2001; Man and Wiktorsson, 2002; Khang, 2004; Khang and Wiktorsson, 2004). The proteolytic activities were merely restricted when the pH of the fermented silage is 4.3 or lower (Carpintero et al., 1979) and in good silage the process will stop earlier and limit the loss of protein (Man and Wiktorsson, 2002).
Morrison (1979) reported that 10-20 % of the hemicelluloses were solubilized during a 150 day ensiling period. A higher ratio of hemicellulose reduction (25 %) was reported by Nishino et al. (1999) while Man and Wiktorsson (2002) obtained a reduction of around 24 % in ensiled cassava tops. The reduction of NDF with molasses addition was due to non-NDF content of molasses. Cyanide and tannin contents were reduced gradually after ensiling. A similar HCN reduction was found by Hang (1998) and Man and Wiktorsson (2001) in cassava foliage and Phuc et al. (2000) in dried cassava foliage. Sun-drying and boiling are simple methods to reduce HCN content, due to the action of endogenous linamarase on glucosides following loss of cell integrity or tissue damage (Man and Wiktorsson, 2002). In the handling and ensiling process in this study, slight wilting during the preparation, pressing and the initial environment of the aerobic phase created good conditions for reducing the HCN. When the pH in the silage was lowered, the enzyme activities were restricted and this reduced the speed of HCN elimination during storage.
The tannin contents of the silage materials were lower than values reported by Man and Wiktorsson (2002) and values of 27.4 g/kg DM obtained by Khang and Wiktorsson (2004). The contents were reduced in the period of ensiling, and may relate to the formation of tannin-protein complexes. Maldonado et al. (1995) reported that insoluble tannin and plant leaf protein complex was established in the pH range of 3.5-5.5. In ensiling sorghum, Rodriguez et al. (1998) reported that tannin concentration decreased with increase in the duration of fermentation. In the present study, the reduction was not related to duration of fermentation but type of additive. Additives have significant effects on the fermentation parameters of ensiled cassava leaves. pH values were within the range and even lower than values of between 4.21 and 4.29 obtained by Man and Wiktorsson (2002) and values of 3.57 and 3.60 obtained by Pinho et al. (2004) for non-wilted and wilted cassava silage respectively. Values obtained for the ensiled materials were within the pH values of below 4.5 considered to be good by Petterson (1988) and that silage with a pH lower than 4.6 can be considered excellent (Ruiz and Ruiz, 1990). The buffer capacity (BC) of materials for ensiling is one of the characteristics that determine the passage from an initial butyric fermentation to the quick establishment of lactic fermentation organisms, which confer desirable characteristics to the silage (Pinho et al., 2004). The BC obtained for the ECF (2,087 mmol kg-1 DM), ECFM (1,808 mmol kg-1 DM) and ECFP (1,827 mmol kg-1 DM) cassava leaves allowed for a fast decline in pH in the ensiled mass, inhibiting acetic and butyric fermentation. Values obtained here were close to values of 2,045 and 1,905 mmol kg-1 DM obtained for non-wilted and wilted aerial part of cassava by Pinho et al. (2004). They are also very similar to those for maize plants (Creste, 2000).
Ammonia nitrogen (NH3-N) works as an important indicator of proteolytic activity during the fermentation process. The concentration of NH3 - N was 8.81 % of the total N in ECF, 6.89 % in ECFM and 12.48 % in ECFP. The ECF silage can be considered excellent, since it showed an ammonia-nitrogen concentration of up to 8 % (Ruiz and Ruiz, 1990). According to Silveira (1975), the ammonia concentrations must not be higher than 12 % of total nitrogen in well preserved silages. The NH3 - N found in ECFP silage was slightly higher and provides evidence that, even though the material had low soluble carbohydrate concentration as contained in the NFE and cellulose composition, a low buffer capacity and an adequate dry matter concentration, degradation of amino acids by bacteria of the genus Clostridium may occur. Nussio and Manzano (1999) indicated that the action of proteolytic enzymes can occur when the material has a reduced rate of dehydration, as is the case of the ensiled cassava leaves.
The addition of molasses and poultry manure increased the concentration of organic acid found in the silages. Lactic acid concentrations were 4.16, 5.13 and 6.91 for ECF, ECFM and ECFP, respectively. These values fall within the range of 3 to 13 % of lactic acid l proposed by Catchpool and Henzel (1971) in silage juice. Addition of molasses and poultry manure increased the performance of lactic acid producing bacteria by about 18.91 and 39.80 % respectively thus indicating higher fermentation efficiency than the control. The acetic acid concentrations were 0.46, 0.58 and 0.70 % for ECF, ECFM and ECFP respectively. These values were higher than values obtained by Man and Wiktorsson (2002) and values of 0.23 and 0.33 % reported by Pinho et al. (2004) for non-wilted and wilted aerial parts of cassava silage.
The butyric acid concentration is an important indicator of proteolytic activity in the materials; it was 0.18, 0.25 and 0.58 % in ECF, ECFM and ECFP respectively. This is contrary to the findings of Woolford (1984) who reported that a lower activity of bacteria of the genus Clostridium occurs in silages with a high dry matter concentration. Pinho et al. (2004) obtained values of 0.55 and 0.49 % for non-wilted and wilted cassava silages respectively. The butyric acid concentrations of 0.25 and 0.58 % in ECFM and ECFP were above the 0.2 % concentration of DM reported by Silveira (1975) as the limit for good quality silage. The silage from ECFM and ECFP were classified as non-satisfactory with regard to butyric acid concentrations (Lavezzo, 1985).
Dry matter intake from silage obtained in this study compared favourably with values of 249 g/d of ensiled cassava leaves fed to goats in Cambodia (Bunyeth and Preston, 2006) while Seng Sokerya and Preston (2003) obtained values of 18.9 to 53 % DM for feed intake. The high dry matter intake may be attributed to a preference for moist (ensiled leaves) rather than dry feeds as observed by Bunyeth and Preston (2006). The CP intake from silage was higher than values of 60.9 g/d obtained by Bunyeth and Preston (2006) and values of between 41 to 55.5 g/d reported by Seng Sokenya and Preston (2003). The high CP intake recorded in ECFM was due to the high DM intake of the animals on this treatment. According to Preston and Leng (1987), additional protein and energy is required by animals to maintain an efficient rumen ecosystem that will stimulate nutrient intake and improve animal performance. Alberto et al. (1993) reported pH values of between 6.77 and 6.99 for leaves of Enterolobiumwhile Ly et al. (2003) obtained pH values of 7.18, 7.01 and 7.05 for cassava leaves in trough, foliage in trough and foliage hanging respectively.
The increase in ruminal NH3-N in ECFP might be due to the contribution of poultry manure as a non-protein nitrogen source. Ly et al. (2003) obtained values of 736 mg/ L, 806 mg/L and 467mg/L for cassava leaves in trough, foliage in trough and foliage hanging, respectively. Alberto et al. (1993) reported ruminal NH3-N of 26.4 and 25.2 (mg N/100 ml) for sheep fed 100 and 300g of E. cyclocarpum while Khang (2004) reported values ranging from 10.41 to 16.25 mg 100 g-1 of ruminal fluid in cattle fed increased levels of cassava foliage. The total volatile fatty acids obtained in this study were higher than values of 80.9 to 93.4 mmol L-1 of ruminal fluid for cattle fed increased levels of cassava foliage by Khang (2004). The ruminal NH3-N concentration had a good profile, with values between a minimum of 2 mg 100ml-1 (Satter and Slyter, 1974) and a maximum of30 mg 100 ml-1 (Srinivas and Gupta, 1997) suggested for maximum microbial growth in the rumen. Leng et al. (1977) reported optimum rumen ammonia concentration of 5-20 mg NH3/100 mL. Low pH can also be caused by an accumulation of VFA in the rumen (Hussain and Cheeke, 1995; Lana et al., 1998). The high total VFA obtained in ECFM might therefore be as a result of the low pH of this material.
Conclusion
Results from this study indicated that ensiling cassava leaves with either molasses or caged layer waste improved the ensiling condition and promote positive effects on rumen functions of West African Dwarf goats.
References
1. AOAC. 2000. Association of Official Analytical Chemists. Official Method of Analysis. 21st Edition. Washington DC. [ Links ]
2. Alberto, G.; Portela, J.S. e Oliveira, O.L.P. 1993. Efeito da adicào de grào de sorgo moido e do emurchecimento sobre a qualidade de silagem de capim-elefante (Penissetum purpureum, Schum.). Rev Soc Bras Zootecn, 22: 1-11. [ Links ]
3. Bunyeth, H. and Preston, T.R. 2006. Growth performance and parasite infestation of goats given cassava leaf silage or sun-dried cassava leaves, as supplement to grazing in lowland and upland regions of Cambodia. LRRD. Vol. 18, Article #28. Retrieved August 24, 2007. http://www.cipav.org.co/lrrd18/2/buny/8028.htm. [ Links ]
4. Carpintero, C.M.; Henderson, A.R. and McDonald, P. 1979. The effect of some pre-treatments on proteolysis during the ensiling of herbage. Grass Forage Sci, 40: 85-92. [ Links ]
5. Catchpoole, V.R. and Henzel, E.F. 1971. Silage and silage-making from tropical herbage species. Herbage Abstracts, 41: 213-221. [ Links ]
6. Creste, C.R. 2000. Potencial para ensilagem, composição bromatológica e qualidade da silagem de milho com diferentes proporções de espigas. UNESP/FMVZ. Botucatu. 27 pp. [ Links ]
7. FAOSTAT. 2012. Food and Agriculture Organization of the United Nations. FAOSTAT. http://faostat3.fao.org/home/index.html#DOWNLOAD. [ Links ]
8. Hang, D.T. 1998. Ensiled cassava leaves and duck weed as protein sources for fattening pigs on farms in Central Vietnam. Livest Res Rural Dev, 10: 3. [ Links ]
9. Hussain, I. and Cheeke, P.R. 1995. Effect of dietary Yucca schidigera extract on rumen and blood profiles of steers fed concentrate or roughage based diets. Anim Feed Sci Technol, 51: 231-242. [ Links ]
10. Khang, D.N. 2004. Cassava foliage as a protein source for cattle in Vietnam. Doctoral thesis. Agraria 471. Swedish University Agric. Sci. Uppsala, Sweden. [ Links ]
11. Khang, D.N. and Wiktorsson, H. 2004. Effects of fresh cassava tops on rumen environment parameters, thyroid gland hormones and liver enzymes of local yellow cattle fed urea-treated fresh rice straw. Trop Anim Health Prod, 36: 751-762. [ Links ]
12. Lana, R.P.; Russel, J.B. and Van Amburgh, M.E. 1998. The role of pH in regulating ruminal methane and ammonia production. J Anim Sci, 76: 2190-2196. [ Links ]
13. Lanyansunya, T.P.; Wang, H.; Kariuki, S.; Mukisira, E.; Abdulrazak, S.; Kibitok, N. and Ondiek, J. 2007. The potential of Commelina benghalensis as forage for ruminants. Anim Feed Sci Tech, 144: 185-195. [ Links ]
14. Lavezzo, W. 1985. Silagem de capim-elefante. Informe Agropecuário, 11: 50-57. [ Links ]
15. Leng, R.A.; Kempton, T.J. and Nolan, J.V. 1977. Non-protein nitrogen and bypass protein in ruminant diets. AMRC Review, 33: 785-790. [ Links ]
16. Limon Loeza, R.L. 1992. Ensilage of cassava products and their use as animal feed. In: Roots, tubers, plantains and bananas in animal feeding. D. Machin and Solveig Nyvold (Eds.). FAO Animal Production and Health Paper, 95: 99-110. http://www.fao.org/ag/aga/agap/frg/AHPP95/95-99.pdf. [ Links ]
17. Ly, J.; Theng, K. and Preston, T.R. 2003. Studies on utilization of trees and shrubs as the sole feedstuff by growing goats: foliage preferences and nutrient utilization. Livest Res Rural Dev, 15(7). [ Links ]
18. Maldonado, R.A.P.; Norton, B.W. and Kerven, G.L. 1995. Factors affecting in vitro formation of tannin-protein complexes. J Sci Food Agric, 69: 291-298. [ Links ]
19. Man, N.V. and Wiktorsson, H. 2001. Cassava tops ensiled with or without molasses as additive effects on quality, feed intake and digestibility by heifers. Asian-Aust J Anim Sci, 14: 624-630. [ Links ]
20. Man, N.V. and Wiktorsson, H. 2002. Effect of molasses on nutritional quality of cassava and gliricidia tops silage. Asian-Aust J Anim Sci, 15: 1294-1299. [ Links ]
21. Morrison, I.M. 1979. Changes in the cell wall components of laboratory silages and the effect of various additives on these changes. J Agric Sci Camb, 93: 581-586. [ Links ]
22. Nguyen Thi Loc.; Ogle, R.B. and Preston, T.R. 1996. On farm and on station evaluation of cassava root silage for fattening pigs in Central Vietnam. MSc. Thesis. Swedish University of Agricultural Sciences. [ Links ]
23. Nishino, N.; Sasaki, A. and Uchida, S. 1999. Impact of ensiling on cell wall carbohydrates assessed by chemical analysis and ruminal fibrolytic enzyme activity. The XIIth International Silage Conference. Uppsala, Sweden. July 5-7. pp. 237-238. [ Links ]
24. Nussio, L.G. 1991. Milho e sorgo para producao de silagem. Producao de alimentos volumosos parabovinos. FEALQ. Anais Piracicaba. pp. 75-177. [ Links ]
25. Nussio, L.G. e Manzano R.P. 1999. Valor nutritivo e conservação. Simpósio sobre manejo da pas-tagem: Alfafa. Anais. FEALQ. Piracicaba. pp. 153-173. [ Links ]
26. Petterson, K. 1988. Ensiling of forages. Factors affecting silage fermentation and quality. Dissertation, Swedish Univ. of Agric. Sci. Report 179. Uppsala, Sweden. [ Links ]
27. Phuc, B.N.H.; Ogle, B. and Lindberg, J.E. 2000. Effect of replacing soybean protein with cassava leaf protein in cassava root meal based diets for growing pigs on digestibility and N retention. Anim Feed Sci Techno!, 83: 223-235. [ Links ]
28. Pinho, E.Z.; Costa, C.; Arrigoni, M.D.B.; Silveira, A.C.; Padovani, C.R. and Pinho, S.Z. 2004. Fermentation and nutritive value of silage and hay made from the aerial part of cassava (Manihot esculenta Crantz). Sci Agric, 61: 364-370. [ Links ]
29. Preston, T.R. and Leng, R.A. 1987. Matching ruminant production systems with available resources in the tropics and sub-tropics. Penambul Books Armidale. Queensland. Australia. pp. 117-118. [ Links ]
30. Ravindran, V. and Rajaguru, A.S. B. 1988. Effect of stem pruning on cassava root yield and leaf growth. Sri Lankan J Agric Sci, 25: 32-37. [ Links ]
31. Rodriguez, N.M.; Borges, A.L.C.C.; Goncalves, L.C.; Zago, C.P. and Lara, A.C. 1998. Forage sorghum silages with different tannin and moisture contents in the stem. III. Effects on nitrogenous compounds. Arq Bras Med Vet Zoo, 50: 161-165. [ Links ]
32. Ruiz, E.M. e Ruiz, A. 1990. Metodologías para investigaciones sobre conservación y utilización de silagens. Instituto Interamericano de Cooperación para la Agricultura. Nutrición de ruminantes: guia metodológico de cooperación. IICA. San José. pp. 179-218. [ Links ]
33. Satter, L.D. and Slyter, L.L. 1974. Effect of ammonia concentration on rumen microbial protein production in vitro. Brit J Nutr, 32: 199-208. [ Links ]
34. Sokerya, S. and Preston, T.R. 2003. Effect of grass or cassava foliage on growth and nematode parasite infestation in goats fed low or high protein diets in confinement. Livest Res Rural Dev, (15)8. http://www.cipav.org.co/Lrrd15/8/kery158.htm. [ Links ]
35. Silveira, A.C. 1975. Técnica para produção de silagem. Simpósio sobre manejo de pastagens, 2. Anais. FEALQ. Piracicaba. pp. 156-186. [ Links ]
36. SPSS. 1999. Statistical Package for Social Sciences. Procedures and facilities for release. McGraw-Hill Book Co. NY. [ Links ]
37. Srinivas, B. and Gupta B. 1997. Rumen fermentation, bacterial and total volatile fatty acids (TVFA) production rates in cattle fed on urea-molasses-mineral block licks supplements. Anim Feed Sci Tech, 65: 275-286. [ Links ]
38. Terrill, T.H.; Rowan, A.M.; Douglas, G.B. and Barry, T.N. 1992. Determination of extractable and bound condensed tannin concentrations in forage plants, protein-concentrate meals and cereal grains. J Sci Food Agric, 58: 321-329. [ Links ]
39. Tewe, O.O. 1991. Detoxification of cassava products and effects of residue toxins on consuming animals. In: Roots, tubers, plantains and bananas in animal feeding. D. Machin and Solveig Nyvold (Eds.). FAO Animal Production and Health Paper, 95: 81-95. http://www.fao.org/ag/aga/agap/frg/AHPP95/95-81.pdf. [ Links ]
40. Van Soest, P.J.; Roberson, J.B. and Lewis, B.A. 1991. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci, 74: 3583-3597. [ Links ]
41. Wiseman, H.G. and Irvin, H.M. 1957. Determination of organic acids in silage. J Agric Food Chem, 5: 213-215. [ Links ]
42. Woolford, M.K. 1984. The silage fermentation. Marcel Dekker. NY. 350 pp. [ Links ]
Recibido: 14-8-12.
Aceptado: 19-6-14.