My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nefrología (Madrid)
On-line version ISSN 1989-2284Print version ISSN 0211-6995
Nefrología (Madr.) vol.30 n.1 Cantabria 2010
Prevalence of chronic renal disease in Spain: Results of the EPIRCE study
Prevalencia de la insuficiencia renal crónica en España: Resultados del estudio EPIRCE
Alfonso Otero González1, A. de Francisco2, P. Gayoso1, F. García3, on behalf of the EPIRCE Study Group
1Nephrology Department and Research Unit. Ourense Hospital Complex. Orense (Spain)
2Nephrology Department. Hospital Marqués de Valdecilla. Santander (Spain)
3Clinical Epidemiology Unit. University Hospital Puerta de Hierro. Majadahonda, Madrid (Spain)
This study was supported in part by a grant from Amgen, S.A. Writing assistance was supported by Amgen.
ABSTRACT
Introduction: Chronic kidney disease (CKD) is an independent cardiovascular risk factor. The knowledge of prevalence in general population may help to early detection of CKD and prevent or delay its progression.
Methods: Sociodemographic, baseline characteristics, and CKD prevalence (measured by centralized serum creatinine and MDRD equation) were evaluated in a randomly selected sample of general population aged 20 years or older, collected in all Spanish regions and stratified by habitat, age and sex according to 2001 census (n = 2,746). Univariate and multivariate logistic regression analyses were used to evaluate associations with CKD risk factors.
Results: Mean age was 49.5 years. The overall prevalence of Kidney Disease Outcomes Quality Initiative grades 3-5 CKD was 6.8%, with a 95% confidence interval (CI) of 5.4 to 8.2 (3.3% for age 40-64 years and 21.4% for age >64 years). The prevalence estimates of CKD stages were: 0.99% for stage 1 (glomerular filtration rate [GFR] >90 ml/min per 1.73 m2 with proteinuria); 1.3% for stage 2 (GFR 60-89); 5.4% for stage 3a (GFR 45-59); 1.1% for stage 3b (GFR 30-44); 0.27% for stage 4 (GFR 15-29); and 0.03% for stage 5 (GFR <15). An important prevalence of classical cardiovascular risk factors was observed: dyslipemia (29.3%), obesity (26.1%), hypertension (24.1%), diabetes (9.2%) and current smoking (25.5%). The independent predictor factors for CKD were age, obesity and previously diagnosed hypertension.
Conclusions: The prevalence of CKD at any stage in general population from Spain is relatively high, especially in the elderly, and similar to countries of the same geographical area. Independently of age, two modifiable risks factors, hypertension and obesity, are associated with an increased prevalence of CKD.
Key words: Cardiovascular risk factors, Chronic kidney disease, Epidemiology.
RESUMEN
Introducción: La insuficiencia renal crónica (IRC) constituye un factor de riesgo cardiovascular independiente. El conocimiento de su prevalencia en la población general puede contribuir a la detección precoz de esta enfermedad y de prevenir o retrasar su evolución.
Métodos: Se seleccionó una muestra aleatoria de población general española, con edad igual o superior a 20 años, distribuida por todo el territorio nacional y estratificada por hábitat, edad y sexo conforme al censo de 2001 (n = 2.746). Se recopilaron datos sociodemográficos y clínicos, y se evaluó la prevalencia de IRC mediante determinación centralizada de creatinina sérica y aplicación de la ecuación MDRD. Se llevaron a cabo análisis univariantes y multivariantes para evaluar la asociación entre la IRC y diversos factores de riesgo.
Resultados: La edad media fue de 49,5 años. La prevalencia global de IRC en estadios 3-5, según la Kidney Disease Outcomes Quality Initiative, fue del 6,8%, con un intervalo de confianza del 95% (IC) de 5,4 a 8,2 (3,3% para edades 40-64 años y 21,4% para edades >64 años). Las prevalencias estimadas para cada uno de los estadios de IRC fueron: 0,99% para estadio 1 (tasa de filtrado glomerular [TFG] >90 ml/min por 1,73 m2 con proteinuria); 1,3% para estadio 2 (TFG 60-89); 5,4% para estadio 3a (TFG 45-59); 1,1% para estadio 3b (TFG 30-44); 0,27% para estadio 4 (TFG 15-29), y 0,03% para estadio 5 (TFG <15). Se apreció una prevalencia considerable de factores de riesgo cardiovascular clásicos: dislipemia (29,3%), obesidad (26,1%), hipertensión (24,1%), diabetes (9,2%) y tabaquismo activo (25,5%). Los factores predictores independientes de IRC fueron la edad, la obesidad y la hipertensión previamente diagnosticada.
Conclusiones: La prevalencia de IRC (en cualquier estadio) en la población general española es relativamente elevada, en especial en los individuos de edad avanzada, y similar a la de otros países del mismo entorno geográfico. Además de la edad, dos factores de riesgo modificables, la hipertensión y la obesidad, se asociaron con una mayor prevalencia de IRC.
Palabras clave: Factores de riesgo cardiovascular, Insuficiencia renal crónica, Epidemiología.
Introduction
Chronic kidney disease (CKD) is a major social health problem. In the last decade, it has been shown that early stages of CKD are asociated with an inflammatory state1 that implies an increased cardiovascular morbidity and mortality risk at long term2,3, higher than the risk of progression to end-stage renal disease2,4. Cardiovascular events are the most common cause of death in these patients5. For this reason, microalbuminuria and reduced glomerular filtration rate (GFR) (<60 ml/min) have been added to the list of non traditional cardiovascular risk factors6. In many patients, the concurrence of these markers with classical factors as diabetes, hypertension or obesity, predicts accelerated vascular damage and multiplies the associated risk2,3.
Furthermore, the prevalence of CKD is growing worldwide due to the increase in related diseases as type 2 diabetes mellitus, obesity, hypertension or atherosclerosis7,8. The asymptomatic nature of CKD makes its early detection more difficult, which could be important as the treatment in early stages may prevent or delay its progression9. The knowledge of the prevalence of CKD might be useful to assess the level of its underdiagnosis and estimate the impact of potential screening policies.
The 2002 practice guideline of the Kidney Disease Outcomes Quality Initiative (K/DOQI) of the National Kidney Foundation (NKF)10 defined CKD as either kidney damage or glomerular filtration rate (GFR) below 60 ml/min/1.73 m2 for three or more months. GFR is usually estimated from serum creatinine using one of the following equations: the Cockcroft-Gault (CG)11 or the Modification of Diet in Renal Disease Study (MDRD)12 equation. These indirect methods are currently considered to be the easiest way to estimate GFR in epidemiologic studies conducted in adult individuals13. The MDRD equation is more commonly used14, but it leads to a certain underestimation of GFR (6.2% in CKD patients and 29% in healthy persons)15, compared to the CG equation. However, it seems that the MDRD equation provides a more accurate estimation in patients with GFR below 60 ml/min/1.73 m2, with good performance among subgroups of age, sex, race, diabetes or body mass index16,17.
In the last five years, more than 25 epidemiological studies have investigated CKD prevalence worldwide14, leading to a median prevalence of 7.2% in persons aged 30 years or older, and revealing ethnic-specific differences. In our country, the Spanish Society of Nephrology (S.E.N.) has initiated a program to identify the true population at risk for CKD, and to increase the preventive measures aimed at reducing the incidence of renal failure, cardiovascular complications, and progression to end stage renal failure18,19.
Within this program, the "Estudio Epidemiólogico de la Insuficiencia Renal en España" (EPIRCE) is the first epidemiological study at a national level designed to describe the prevalence of CKD in the general Spanish population aged 20 years or older, using the simplified MDRD equation.
Methods
The EPIRCE was an epidemiologic, general population-based, cross-sectional study that included a randomly selected Spanish sample aged 20 years or older. The exclusion criteria were residence outside the recruiting municipality, or institutionalization at the time of the study. The protocol was approved by an ethics committee, and all enrolled patients provided informed consent.
The target sample were 13,013 individuals, stratified by age, sex, and habitat within each Spanish region, according to the 2001 Census. A total of 6,464 out of the initial list of 13,013 were finally contacted for the study. Census errors were the most important reason for the impossibility to contact individuals. The sample was recruited between January 2004 and January 2008 in 42 points (municipalities). The final completed interviews were 2,746, and the response rate was 42.5%.
Data were collected as follows. First, a letter describing the study was sent to each randomly selected individual. Next, a health professional contacted the potential respondents by phone to verify inclusion and exclusion criteria, ask for participation, and make appointments with those who volunteered. A minimum of three negative answers were required to discard a selected individual.
The collected variables included anthropometric and sociodemographic data (age, gender, ethnicity, weight, height, body mass index), blood pressure and clinical history at study inclusion (obesity, hypertension, diabetes mellitus, dyslipemia, cardiovascular disease, gout, renal lithiasis, CKD, transplant). Participants were also interviewed to determine their smoking and exercise habits, alcohol consumption, drug abuse and use of nephrotoxic drugs. After informed consent was provided, a blood sample was obtained from each individual for biochemical tests. Serum creatinine concentration was determined in the same reference laboratory for all samples. GFR was calculated as an indicator of renal function with the simplified MDRD formula20, and participants were classified according to the Kidney Disease Outcomes Quality Initiative guidelines10. Stage 3 was split into 3a (GFR 45-59 ml/min/1.73 m2) and 3b (GFR 30-44 ml/min/1.73 m2). Other analytical determinations included: glucose, urea, total cholesterol (C), tryglicerydes (Tg), HDL-C, LDL-C, insulin resistance index (HOMA), haemoglobin (Hb), ferritin, uric acid and urinary albumin to creatinine ratio.
Statistical methods
Adjustment weights were used to correct for non-response bias, with the age, gender and habitat distribution of survey respondents being equated to the population structure as determined from the 2001 census. All prevalence and mean estimates were calculated with the weighted sample, and asymptotic 95% confidence intervals (CI) were obtained. Univariate and multivariate logistic regression analyses, also weighted for non-response bias, were used to calculate the odds ratio (OR) and CIs for candidate CKD risk factors. P values <0.05 were considered significant. Since there were statistically significant differences in the response rate between participating municipalities (data not shown), a sensitivity analysis was performed comparing the results between highly responding centers (>60% of response rate, n = 1,098) and the overall group, to assess for a possible non-response bias. All analyses were performed with SAS version 9.1.3 Service Pack 4 (SAS Institute Inc., Carey, North caroline, USA).
Results
Sociodemographic and clinical characteristics
Table 1 and 2 show the characteristics of the 2,746 respondents (weighted estimates). Mean age was 49.5 years, and about one quarter of individuals were older than 64 years (25.8%). As in the general Spanish population, the ratio male:female was 0.9, almost all were caucasian (99.1%), and the residence was urban in two thirds of cases (66.1%).
Table 1. Demographic and clinical characteristics of Spanish population aged 20 years
or older based on the cohort collected in the EPIRCE study (n = 2,746). 
a Frequency estimates calculated on the weighted sample; blog(TG/HDL-C), with TG and HDL-C
expressed in molar concentrations.
SEM: Standard error of the mean; HOMA: Homeostasis Model Assessment index; ACR: urine albumin
to urine creatinine ratio; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure;
GFR: glomerular filtration rate; HDL: high density lipoprotein-cholesterol; LDL: low density lipoprotein; Hb: haemoglobin.
Table 2. Prevalence of chronic kidney disease in the Spanish population aged 20 years
or older based on thee cohort collected in the EPIRCE study (n = 2,746). 
a There were no patients with eGFR <15 ml/min per 1.73 m2 ; GFR: glomerular filtration rate;
b Prevalence estimates calculated on the weighted sample.
Clinical history revealed an important prevalence of previously diagnosed dyslipemia (29.3%), obesity (26.1%), hypertension (24.1%) and diabetes (9.2%). Among cardiovascular events, peripheral vascular episodes were the most frequent (10.8%), followed by ischaemic heart disease (5.1%) and cerebrovascular disease (1.7%). Current smoking habit and habitual alcohol intake were frequent (25.5% and 45.1%, respectively).
CKD prevalence
The overall prevalence of CKD stages 3-5 (eGFR <60 ml/min) was 6.83 %, with a 95% CI of 5.41 to 8.25 (3.33% for age 40-64 years and 21.42% for age >64 years). When the albumin to creatinine ratio was added to the diagnostic criteria, the prevalence rose to 9.16% (95% CI, 7.5 to 10.8). The prevalence estimates of CKD stages were: 0.99% for stage 1; 1.3% for stage 2; 5.4% for stage 3a; 1.1% for stage 3b; 0.27% for stage 4; and 0.03% for stage 5 (table 2). The prevalence of proteinuria (ACR>30 mg/g) in stage 3a was 5.9%, in stage 3b, 6.8%, and in stage 4, 36.7%.
Risk factors for CKD
Table 3 shows the unadjusted associations between sociodemographic and clinical characteristics of the patients and CKD. The strongest predictor factor was age. The observed odds ratios (OR) were 34.4 for individuals between 40-64 years with respect to those between 20-39 years, and 267.5 for individuals above 64 years. Other strong predictor factors were hypertension, especially when previously diagnosed (OR 5.9), pulse pressure above 60 mmHg (OR 3.8), previous history of cardiovascular events (ORs 4.1 for ischaemic heart disease, 3.3 for cerebrovascular disease and 2.1 for peripheral vascular disease), overweight or obesity (ORs of 2.3 and 3.5, respectively), diabetes (OR 2.4 for previously diagnosed patients), dyslipemia (OR 2.1 for previously diagnosedpatients) and gout (OR 2.2).
Table 3. Unadjusted associations between demographic or clinical characteristics
and the presence of chronic kidney disease (eGFR <60 ml/min per 1.73 m2) 
a OR for predicting stages 4-5; For stages 3-5, the OR is 0.81 (95% CI: 0.55 to 1.2).
HOMA: Homeostasis Model Assessment index; ACR: urine albumin to urine creatinine ratio;
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; GFR: glomerular
filtration rate; HDL: high density lipoprotein-cholesterol; LDL: low density lipoprotein; Hb: haemoglobin.
In the multivariate analysis, the independent predictor factors that remained in the model were age, obesity and previously diagnosed hypertension (table 4).
Table 4. Independent predictors of chronic kidney disease (eGFR <60 ml/min per 1.73 m2 )
in the multivariate logistic regression model 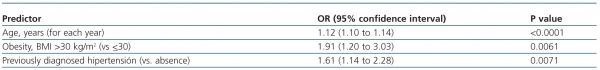
BMI: body mass index; GFR: glomerular filtration rate.
Sensitivity analyses
Individuals recruited at highly responding centers (>60% of response rate, n = 1,098) were healthier according to the following differences with respect to the overall sample: they were less obese (22.9% with BMI >30 kg/m2 versus 26.1% in the total population), less sedentary (25.4% versus 28.9%) and suffered less diabetes (5.2% of non previously diagnosed diabetes versus 7.0%). They also displayed more percentage of habitual alcohol consumption (49.7% versus 45.1%). Despite these findings, the prevalence of CKD stages 3-5 in this subgroup was equivalent to that found in the overall sample: 6.65 (95% CI of 4.66 to 8.64). The prevalence of proteinuria (ACR >30 mg/g) was slightly lower (3.6%, 95% CI 1.4 to 5.8), but not significantly different. No differences were observed either in the prevalences within age, gender or habitat categories, nor in the risk factors associated to CKD (data not shown).
Discussion
The present study is the first epidemiological investigation of the prevalence of CKD in Spanish population aged 20 years or older at a national level. The recruited sample is representative of all regions, and has been adjusted to provide valid estimates of CKD prevalence in age, gender and habitat subgroups, according to the real distribution of Spanish population in 2001.
The prevalence of CKD found in our study (6.8%) is very similar to the median reported in a systematic review of 26 epidemiological studies around the world (7.2%)14. Since ethnic-specific differences have been reported14, the relevant comparisons with other European countries show that the prevalence in Spain remains within the range of previous studies that have used the MDRD equation (4.7-8.1% in studies from Italy21, Switzerland22, Norway23 and Iceland24). These estimates are also similar to those from the US National Health and Nutrition Examination Surveys (NHANES) (5.6% in 1988 through 1994 and 8.05% in 1999 through 2004)25, despite the incidence of end stage renal disease (ESRD) in this country being much higher than in Europe26. The epidemiological study from Norway23 investigated the progression rate from CKD stages 3 or 4 to ESRD in their cohort and found that the relative risk of progression in US caucasian patients was 2.5 times higher than in Norwegian patients. Among the possible explanations for these differences they postulate a later referral to nephrologist and a higher presence of obesity and diabetes in the US population.
The addition of the albumin/creatinine ratio to the CKD diagnosis (stages 1 and 2) allowed to detect a further 2.3% of population at risk, which substantially improves diagnostic accuracy without losing predictive power. According to previous studies, referral based on current stages 3 to 4 CKD identifies approximately only 70% of all individuals that progress to ESRD27.
We found a high prevalence of conventional risk factors, overweight and obesity, hypertension, diabetes, dyslipemia and smoking. All of them were significantly associated to CKD, which agrees with previous findings28,29. With respect to smoking habit, we did not find a significant association with current smoking, but the ex-smoker status was related to a higher frequency of stage 4 CKD. A possible explanation is that, previous to the study entry, these patients had already suffered other health problems that compelled them to discontinue tobacco. Unexpectedly, the habitual alcohol intake was inversely associated to CKD, which partially agrees with the study of Kronborg et al., who found that alcohol consumption in men predicted an increase in eGFR28,29. Red wine has been shown to improve surrogate markers for cardiovascular disease, such as nitric oxid release in the vessel wall. It also possesses anti-inflammatory and anti-oxidative properties, and inhibits platelet-derived growth factor-beta receptor phosphorylation30. However, it would be very difficult to perform prospective, randomized studies to demonstrate the benefits of moderate alcohol consumption, as the important secondary harmful effects (such as liver cirrhosis, blood pressure elevation, cancer or accidents) should be taken into account.
The three independent predictor factors for CKD were increasing age, obesity and history of hypertension, which suggests that these conditions predispose to renal impairment through different mechanisms.
The decline in GFR with age has been repeatedly described14. The prevalence of CKD in patients above 64 years found in the EPIRCE study (21.4%) is comparable to that reported in other European countries (15-25%21-24), usually with higher prevalences in older women22,24. The reduction starts progressively in the third decade of life, and becomes steeper after the age of 60, although it has not been observed in all individuals31. There are several hypotheses to explain this phenomenon: it can be related to pathologic processes (cumulated immunologic, infectious, or toxic damage), progressive ischemia due to vascular aging, or cummulative changes in kidney structure due to hyperperfusion and hyperfiltration with resultant glomerulosclerosis32,33.
The contribution of sustained high blood pressure levels to renal function deterioration is well established: systemic and glomerular hypertension results in increased urinary excretion of proteins and accelerates renal function deterioration. Many studies have demonstrated that an adequate, or even intensified blood pressure control (less than 130/80 mmHg), can slow the progression of diabetic and non diabetic renal disease34. Moreover, long-term studies indicate that the change in GFR may be minimal in well-controlled hypertensive patients, and that patients with nonmalignant essential hypertension with early and good blood pressure control do not develop renal failure35. The relationship found in our cohort might be the result of inadequately controlled blood pressure levels in the individuals with current CKD.
The association between CKD and obesity was previously described in a prospective study of a large cohort36. The increase in body weight with time, even within normal BMI values, has also been independently associated with an increased risk for CKD37. One of the proposed mechanisms for the development of CKD in obese patients is the presence of an increased inflammation status. This is supported by the study of Bavbek et al., who found elevated serum C-reactive protein (CRP) levels in obese patients versus age-matched healthy controls, and a negative correlation between CRP levels and GFR38. In morbidity obese patients who underwent very important weight reduction after biliopancreatic diversion all cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, proteinuria ) improved during follow-up39.
An early identification of CKD in primary care is very important, as specialist referral at an appropriate timing may improve long-term outcomes. It has been reported that, in Spain, late referral to nephrologist is common in chronic diseases such as diabetes or hypertension40. Our results indicate that almost ten percent of adult individuals may suffer some degree of renal impairment, and therefore, reveal the need for taking this disease into account. In addition, our findings suggest that the control of classical cardiovascular risk factors as obesity or hypertension in primary care setting might help preventing CKD development.
The main limitation of the study is its poor response rate. The sensitivity analysis excluding the centers with low participation revealed some non-response bias, which did not appear to introduce substantial bias into CKD and proteinuria prevalence estimates. Another limitation is the indirect GFR estimation method, based on a single creatinine measurement, that should be used with caution41. Currently, the benefit of performing extensive screening of unselected populations with the intention to reduce the subsequent risk of cardiovascular events or progression to end-stage-renal disease remains unproven42. Although the MDRD equation is the most commonly used in epidemiological studies14, it underestimates the GFR15. Moreover, the cut-off value of 60 ml/min per 1.73 m2 for all ages leads to over diagnosis in elderly population. A new equation recently developed seems to improve the GFR estimation43. Finally, the cross-sectional design of the study does not allow inferring causal relationships between the risk factors and CKD.
Some strenghts of our study are its large sample size, well representative of the different Spanish regions, and the random selection of the participants. The agreement with results from other European countries supports the external validity of our findings.
In conclusion, we found a relatively high prevalence of assymptomatic CKD (almost one of ten) in apparently healthy general population from Spain, especially in older, obese and hypertensive patients. Independently of age, many of the risks factors for CKD are modifiable: hypertension, diabetes mellitus, obesity, dyslipemia and smoking. Further studies should assess whether early detection of CKD in general population might avoid CKD progression and protect from associated cardiovascular risk factors.
Acknowledgements
Co-authors. This study has been conducted by the above-signed authors, Neus Valveny from Trial Form Support, David Calbet from Saatchi&Saatchi Healthcare, and the EPIRCE study group.
The Coordinating investigators in each Authonomous Community were: Andalucía: M.A. Álvarez de Lara; Asturias: F. Vega; Aragón: C. Laviades, P.J. Vives, J.M. Peña Porta; Baleares: J. Marco, A. Solís, A. Losada González; Cantabria: G. Fernández Fresnedo; Canarias: J.F. Navarro, J.A. Sánchez Joga; Catalunya: J. Fort, A. Martínez Castelao, N. Fonseré; Castilla-La Mancha: F. Tornero, M. Quintana; Castilla-León: J. Grande Villoria, A. Molina, B. del Pozo, G. Torres; Ceuta y Melilla: C. Fernández Andrade; Euskadi: F. Vidaur, J. Manrique, M. Rodríguez; Extremadura: F. Caravaca, B. Cancho; Galicia: A. Otero, L. González; La Rioja: A. Sánchez Casajús; Madrid: F. García, M. San Boixedau, K. López, E. Rubio, C. Bernis; Murcia: M. Gironés; Navarra: J.L. Asín; Valencia: J. Hernández Jaras, A. Rius, M. González Rico.
References
1. Stenvinkel P, Barany P, Heimburger O, Pecoits-Filho R, Lindholm B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int Suppl. 2002;80:103-8. [ Links ]
2. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154-69. [ Links ]
3. Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet 2001;357:1601-8. [ Links ]
4. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med 2001;134:629-36. [ Links ]
5. Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002;13:745-53. [ Links ]
6. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560-72. [ Links ]
7. Perneger TV, Brancati FL, Whelton PK, Klag MJ. End-stage renal disease attributable to diabetes mellitus. Ann Intern Med 1994;121:912-8. [ Links ]
8. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003;14:2934-41. [ Links ]
9. Locatelli F, Vecchio LD, Pozzoni P. The importance of early detection of chronic kidney disease. Nephrol Dial Transplant 2002;17(Suppl 11):2-7. [ Links ]
10. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1-266. [ Links ]
11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. [ Links ]
12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. [ Links ]
13. Verhave JC, Balje-Volkers CP, Hillege HL, De Zeeuw D, De Jong PE. The reliability of different formulae to predict creatinine clearance. J Intern Med 2003;253:563-73. [ Links ]
14. Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008;8:117. [ Links ]
15. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929-37. [ Links ]
16. Lamb EJ, Tomson CR, Roderick PJ. Estimating kidney function in adults using formulae. Ann Clin Biochem 2005;42:321-45. [ Links ]
17. Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749-57. [ Links ]
18. Martín de Francisco AL, Aguilera L, Fuster V. Cardiovascular, renal and other chronic diseases. Early intervention is necessary in chronic kidney disease. Nefrología 2009;29:6-9. [ Links ]
19. De Francisco AL, De la Cruz JJ, Cases A, De la Figuera M, Egocheaga MI, Gorriz JI, et al. Prevalence of kidney insufficiency in primary care population in Spain: EROCAP study. Nefrología 2007;27:300-12. [ Links ]
20. Levey AGT, Kuseek, JW, the MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000;11:155A. [ Links ]
21. Cirillo M, Laurenzi M, Mancini M, Zanchetti A, Lombardi C, De Santo NG. Low glomerular filtration in the population: prevalence, associated disorders, and awareness. Kidney Int 2006;70:800-6. [ Links ]
22. Nitsch D, Felber D, Von Eckardstein A, Gaspoz JM, Downs SH, Leuenberger P, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 2006;21:935-44. [ Links ]
23. Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006;17:2275-84. [ Links ]
24. Viktorsdottir O, Palsson R, Andresdottir MB, Aspelund T, Gudnason V, Indridason OS. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 2005;20:1799-807. [ Links ]
25. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038-47. [ Links ]
26. López Revuelta K, Saracho R, García López F, Gentil MA, Castro P, Castilla J, et al. Dialysis and Transplant Registry of the Spanish Society of Nephrology and regional registries. Rapport 2001. Nefrología 2004;24:21-6,8-33. [ Links ]
27. Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and Albuminuria to Classify CKD Improves Prediction of ESRD. J Am Soc Nephrol 2009;28. [ Links ]
28. Kronborg J, Solbu M, Njolstad I, Toft I, Eriksen BO, Jenssen T. Predictors of change in estimated GFR: a population-based 7-year follow-up from the Tromso study. Nephrol Dial Transplant 2008;23:2818-26. [ Links ]
29. Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005;80:1270-7. [ Links ]
30. Bohm M, Rosenkranz S, Laufs U. Alcohol and red wine: impact on cardiovascular risk. Nephrol Dial Transplant 2004;19:11-6. [ Links ]
31. Douville P, Martel AR, Talbot J, Desmeules S, Langlois S, Agharazii M. Impact of age on glomerular filtration estimates. Nephrol Dial Transplant 2009;24:97-103. [ Links ]
32. Lamb EJ, O'Riordan SE, Delaney MP. Kidney function in older people: pathology, assessment and management. Clin Chim Acta 2003;334:25-40. [ Links ]
33. Lindeman R. Overview: renal physiology and pathophysiology of aging. Am J Kidney Dis 1990;16:275-82. [ Links ]
34. Pisoni R, Remuzzi G. How much must blood pressure be reduced in order to obtain the remission of chronic renal disease? J Nephrol 2000;13:228-31. [ Links ]
35. Ljungman S. The kidney as a target of hypertension. Curr Hypertens Rep 1999;1:164-9. [ Links ]
36. Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 2005;46:871-80. [ Links ]
37. Ryu S, Chang Y, Woo HY, Kim SG, Kim DI, Kim WS, et al. Changes in body weight predict CKD in healthy men. J Am Soc Nephrol 2008;19:1798-805. [ Links ]
38. Bavbek N, Isik B, Kargili A, Uz E, Uz B, Kanbay M, et al. Association of obesity with inflammation in occult chronic kidney disease. J Nephrol 2008;21:761-7. [ Links ]
39. Palomar R, Fernández-Fresnedo G, Domínguez-Díez A, López-Deogracias M, Olmedo F, Martín de Francisco AL, et al. Effects of weight loss after biliopancreatic diversion on metabolism and cardiovascular profile. Obes Surg 2005;15:794-8. [ Links ]
40. Pérez-García R, Martín-Malo A, Fort J, Cuevas X, Lladós F, Lozano J, et al. Baseline characteristics of an incident haemodialysis population in Spain: results from ANSWER-a multicentre, prospective, observational cohort study. Nephrol Dial Transplant 2009;24:578-88. [ Links ]
41. Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol 2004;15:1316-22. [ Links ]
42. Glassock RJ, Winearls C. Screening for CKD with eGFR: doubts and dangers. Clin J Am Soc Nephrol 2008;3:1563-8. [ Links ]
43. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. [ Links ]
![]() Correspondence:
Correspondence:
Alfonso Otero González,
Nephrology Department and Research Unit,
Ourense Hospital Complex,
Ramón Puga, 52-56, 32005, Orense, Spain
E-mail: alfonso.otero.gonzalez@sergas.es














