My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Nutrición Hospitalaria
On-line version ISSN 1699-5198Print version ISSN 0212-1611
Nutr. Hosp. vol.24 n.3 Madrid May./Jun. 2009
Anti-inflammatory effect of parenteral fish oil lipid emulsion on human activated mononuclear leukocytes
Efecto antiinflamatorio de la emulsión parenteral de lípidos con aceite de pescado en leucocitos mononucleares humanos activados
T. Manzoni Jacintho1, H. Gotho2, M. Gidlund2, C. García Marques1, R. Torrinhas1, M.ª Mirtes Sales3 and D. Linetzky Waitzberg1
University of São Paulo Medical School. Brazil.
1METANUTRI/Laboratório de Fisiologia e Distúrbios Esfincterianos (LIM35).
2Laboratório de Soroepidemiologia e Imunobiologia (LIM38).
3Divisão de citometria do HCFMUSP. Brazil.
This work was supported by research grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (Fapesp-04/04225-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-505633/04-3). The authors thank Fresenius-Kabi for kindly concede the lipid emulsions
ABSTRACT
Background & aim: To compare the effect of fish oilbased (FO) lipid emulsions (LE) for parenteral administration with standard LE and a new FO containing LE composed of four different oils on the antigen presentation and inflammatory variables.
Methods: Phytohemagglutinin (PHA) activated human mononuclear leukocytes were cultured with different LE - Control: without LE; SO: soybean oil; SO/FO: soybean and FO (4:1); MCT/SO: medium chain triglycerides and SO (1:1); MCT/SO/FO: MCT/SO and FO (4:1) and SMOF: a new LE containing FO. Cytokine production was evaluated by ELISA, the expression of antigen-presenting and co-stimulatory surface molecules were analyzed by flow cytometry and lymphocyte proliferation was assessed by H3-Thymidine incorporation, after tetanus toxoid-induced activation.
Results: All LE decreased the HLA-DR and increased CD28 and CD152 expression on monocytes/macrophages and lymphocytes surface (p < 0.05). SO/FO and MCT/SO/FO decreased lymphocyte proliferation (p<0.05). All LE decreased IL-2 production, but this effect was enhanced with MCT/SO/FO and SMOF (p < 0.05). MCT/SO/FO decreased IL-6 and increased IL-10, whereas SO had the opposite effect (p < 0.05).
Conclusion: FO LE inhibited lymphocyte proliferation and had an anti-inflammatory effect. These effects seem to be enhanced when FO is mixed with MCT/SO. SMOF had a neutral impact on lymphocyte proliferation and IL-6 and IL-10 production.
Key words: Lipid emulsion. Soybean oil. Fish oil. Co-stimulatory molecules and antigen presentation.
RESUMEN
Antecedentes & objetivo: Comparar el efecto de las emulsiones lipídicas (EL) basadas en aceite de pescado (AP) para la administración parenteral con las EL estándar y una nueva EL que contiene AP compuesta por cuatro aceites distintos sobre la presentación antigénica y las variables inflamatorias.
Métodos: se cultivaron leucocitos mononucleares activados con fitohemaglutinina (PHA) con diferentes EL - Control: sin EL; AS: aceite de soja; AS/AP: soja y AP (4:1); TCM/AS: triglicéridos de cadena media y AS (1:1); TCM/AS/AP: TCM/AS y AP (4:1) y SMOF: una nueva EL que contiene AP. Se evaluó la producción de citocinas mediante ELISA, se analizó la expresión de moléculas de superficie de presentación de antígeno y co-estimuladoras mediante citometría de flujo y se evaluó la proliferación linfocitaria mediante la incorporación de timidina-H3 tras la activación inducida por el toxoide tetánico.
Resultados: Todas las EL disminuyeron la expresión de HLA-DR y aumentaron la expresión de CD28 y CD152 sobre superficie de monocitos/macrófagos y linfocitos (p < 0,05). La AS/AP y la TCM/AS/AP disminuyeron la proliferación linfocitaria (p < 0,05). Todas las EL disminuyeron la producción de IL-2, pero su efecto se incrementó con las emulsiones TCM/AS/AP y SMOF (p < 0,05). La TCM/AS/AP disminuyó la IL-6 y aumentó la IL-10, mientras que el AS tuvo el efecto opuesto (p < 0,05).
Conclusión: La EL AP inhibió la proliferación linfocitaria y tuvo un efecto antiinflamatorio. Estos efectos parecen estar potenciados cuando el AP se mezcla con TCM/AS. La SMOF tuvo un efecto neutro sobre la proliferación linfocitaria y la producción de IL-6 e IL-10.
Palabras clave: Emulsión lipídica. Aceite de soja. Aceite de pescado. Moléculas co-estimuladoras y presentación antigénica.
Introduction
Fatty acids can modulate the immune and inflammatory response in in vitro and in vivo studies.1-3
Patients with indication for parenteral nutrition receive fatty acids (FA) as lipid emulsions (LE) for parenteral administration. Depending on the fatty acids composition, LEs can have different impacts on immune functions, and thus affect the patient's clinical course.4-7
The immune response triggered by antigen presentation involves the active participation of different cell-surface molecules with immune functions.8,9 The antigens that undergo phagocytosis by monocytes/macrophages and other antigen-presenting cells are expressed on the cell surface by MHC class II molecules (also known as HLA molecules) because they are recognized by T-helper lymphocytes.8,9
Additionally, the effective activation of the T-helper lymphocytes also depends on the signal triggered by the interaction between the co-stimulatory molecules -CD80 and CD86- found on the surface of monocytes/macrophages, and their receptor CD28.10,11 Without the second co-stimulatory signal, the T-helper lymphocyte does not recognize the antigen as a stimulant and develops immune tolerance.12
After being activated, the immune cells start to produce several pro- and anti-inflammatory cytokines that mediate the immune response against pathogenic agents.
The activation level of the T-helper lymphocytes is controlled by the surface molecule CD152, an alternative ligand for CD80 and CD86 molecules. The interaction between CD152 and the co-stimulatory molecules CD80 and CD86, inhibits the cell activation cascade, preventing enhancement of the immune response.13,14
The new goals of parenteral nutrition therapy for the critically ill include mitigation of inflammation and modulation of the immune system via immunomodulating nutrients, such as n-3 polyunsaturated fatty acids (n-3 PUFA). The n-3 PUFA have shown reduced inflammatory effects and clinical benefits such as shorter hospital stays and a potential decrease in morbidity and mortality rates.15-17
Therefore, it is worth studying the in vitro effects of parenteral lipid emulsions containing fish oil, rich in n-3 PUFA, on the expression of human mononuclear leukocytes surface molecules that participate in the antigen-presentation process, cytokine production and lymphocyte proliferation.
Materials and methods
Ethical statement
All experimental procedures of the current study were previously approved by the local Ethical Scientific Committee.
Selection of volunteers and collection of mononuclear cells
Samples of peripheral blood were drawn from volunteers - healthy male donors (n = 10), 20-40 years of age, non-smokers, mild exercise activity (less than twice a week), non-drinkers of alcohol, non-users of drugs and with history of no disease condition near (3 weeks) the date blood was collected.
Mononuclear cells were obtained by the Ficoll Hypaque gradient method (Histopaque 1077, Sigma - USA). They were then resuspended in a RPMI medium (RPMI 1640, Gibco-USA), containing 2 mmol/L Lglutamine, 25 mM/L Hepes medium, 0.07 mM/L gentamicin and 1x105 U/L penicillin with 10% inactivated FBS (Fetal Bovine Serum) (Gibco-USA).
Lipid Emulsions and Study Groups
The lipid emulsions available on the market and used in this study have already been described.5
Six groups were established based on the type of lipid emulsion added to the culture medium (table I).
Cell cultures to evaluate the expression of surface molecules, determine the lipid profile of the cell membrane and the production of cytokines
Costar 24-well cell culture plates were used to incubate 2x106 mononuclear cells with viability above 95% (as accessed by Tripan Blue exclusion) under humid atmosphere, with 5% of CO2 at 37 ºC for 24, 48 and 72 hours in 2 mL RPMI 1640 culture medium (Gibco-USA), containing 10% of inactivated FBS (Gibco-USA), 2 mmol/L L-glutamine, 25 mM/L Hepes, 0.07 mM/L gentamicin and 1x105 U/L penicillin, in 10 μg/mL phytohemagglutinin (Phytohemagglutinin = PHA) (Sigma-USA). Different parenteral LEs were added at the concentration of 1 mg/mL to make up the six experimental groups.
Separation of mononuclear cell membranes and gas chromatography
The monocytes/macrophages and lymphocytes of each experimental group were submitted to five cycles of freezing/defreezing in liquid nitrogen. Then the pool of cells of each group was centrifuged at 5,000 rpm for five minutes at 4 ºC. The supernatant was collected and centrifuged for 60 minutes at 30,000 rpm at 4 ºC.18
Then, the fatty acids of the cell membrane were extracted and sterilized using the Folch method.19 Membrane composition was determined by gas chromatography (Shimadzu CG2010AF-Japan), equipped with a splint/splintless injector, flame ionization detector (FID) and a fused silica capillary column - 100 m long, 0.25 μm internal diameter and 0.20 μm film. (Omegawax 2560, Supelco-Japan).
A 37-fatty acid standard was used (FAME Standard Supelco 37-Component FAME Mix, Supeco-Japan). One mL of the sample was analyzed in the splintless mode at 270 ºC, column with initial temperature fixed at 60 ºC with temperature ramp rate of 3.5 ºC/min until it reached 240 ºC. The detector temperature was maintained at 300 ºC.
Analysis of the expression of surface molecules by flow cytometry
Forty-eight hours later, the cells that had not adhered to the culture plate were discarded and only adherent monocytes/macrophages were labeled with the following anti-human monoclonal antibodies: CD14 APC, HLA-DR Cy-Chrome, CD80 FITC and CD86 PE (BD Biosciences, USA) and their respective isotype controls: mouse IgG2a APC, mouse IgG2a,k Cy-Chrome, mouse IgG1 FITC and mouse IgG2b,k PE (BD Biosciences, USA). Seventy-two hours later, the non-adherent cells were labeled with monoclonal antibodies specific to Thelper lymphocytes - anti-human CD3 Cy-Chrome, antihuman CD4 APC, anti-humanCD28 FITC and antihumanCD152 PE (BD Biosciences, USA) and their respective isotype controls - mouse IgG1,k cy-chrome, mouse IgG1,k APC, mouse IgG1 FITC and mouse IgG2a PE (BD Biosciences, USA). After being labeled, the cells were examined by flow cytometry (FacsCalibur Becton & Dickson-USA). Aliquots of monocytes/macrophages incubated for 48 hours and lymphocytes cultured for 72 hours were collected for determining the lipid profile of the cell membrane.
Analysis of cytokine production
To measure cytokine production, samples of the mononuclear cells cultured with the different parenteral LEs and activated with PHA were collected after 24-hours to analyze the production of IL-2 and IL-6 and after 48-hours for IL-10. The samples were stored in liquid nitrogen immediately after collection for later measurement of cytokine production by ELISA, using specific kits (Human IL-2 ELISA Set, Human IL-6 ELISA Set and Human IL-10 ELISA Set, BD Biosciences-USA).
Cell culture to assess lymphocyte proliferation stimulated by antigen-presentation
After obtaining mononuclear cells using the Ficoll Hypaque gradient (Histopaque 1077, Sigma-USA), 3 samples of 2 x 106 mononuclear cells with viability above 95% were incubated for 120 hours in 96-well culture plates (Costar-USA), under humid atmosphere conditions with 5% CO2 at 37ºC, in RPMI 1640 culture (Gibco-USA) containing 10% inactivated BFS (Gibco-USA), 2 mmol/L L-glutamine, 25 mM/L Hepes, 0.07 mM/L gentamicin and 1 x 105 U/L penicillin. One mg/mL of the different ELs was added to the study groups. For stimulating antigen-induced lymphocyte proliferation, 10 μg/mL of tetanus toxoid (TT) (Instituto Butantã, Brazil) were added to the culture. Twelve hours before harvesting, 5 μCi of H3-Thymidine (Amersham Pharmacia, USA) were added. After the end of the reaction, beta counter (Packard, USA) determined the incorporation of H3-Timidine by proliferating cells.
Statistical analysis
Statistical analysis of the data regarding intensity and fluorescence percentage was performed with Friedman's test and Student-Newman-Keuls post-test, with P ≤ 0.05.
The results obtained for lymphocyte proliferation and cytokine production were submitted to statistical analysis using analysis of variance (ANOVA) and the Student-Newman-Keuls test, with P ≤ 0.05.
Results
Lipid profile of the monocyte/macrophage membrane and human lymphocytes treated with different lipid emulsions
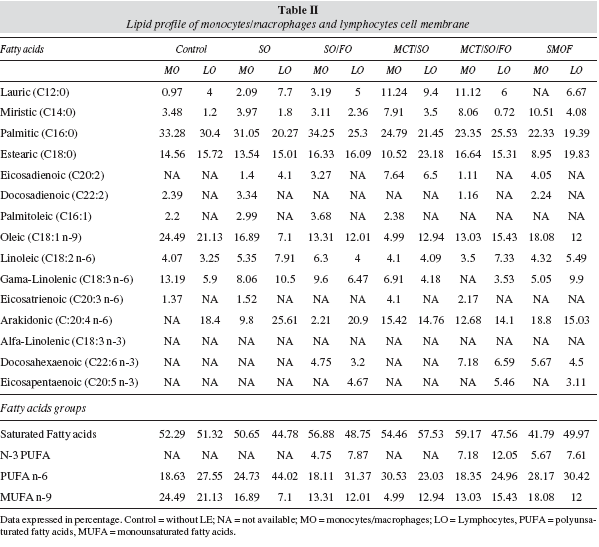
Human monocytes/macrophages -PHA-activated and cultured with different LEs- presented less monounsaturated fatty acids (MUFA) and a higher PUFA concentration at the cell membrane compared to the control group cultured with no lipid emulsion added to it. In the groups cultured with fish oil lipid emulsion, there was an increase in the concentration of n-3 PUFA (docosahexaenoic acid) at the membrane of those cells compared to the control and all the other groups cultured without fish oil.
At the cell membrane of human lymphocytes, there was a decrease in the MUFA n-9 and saturated fatty acids and an increase in PUFA concentration, except for the MCT/SO group, which presented a reduction in n-9 MUFA, n-6 PUFA and an increase in saturated fatty acids. Fish-oil groups had an increase in n-3 PUFA (eicosapentaenoic and docosahexaenoic acids - EPA and DHA) at the cell membrane of lymphocytes compared to the control and to other groups cultured without fish oil (table II).
Effect of LEs on the expression of surface molecules
All parenteral lipid emulsions, regardless of the fatty acid content, reduced the fluorescence intensity of the antigen-presenting molecules HLA-DR expression on human monocytes/macrophages surface, and enhanced the fluorescence (%) of human T-helper lymphocytes expressing CD28 and CD152 surface molecules. The different LEs did not affect the expression of CD80 and CD86 molecules on the surface of human monocytes/macrophages (table III).
Effect of LE on lymphocytes capacity to proliferate via antigen presentation
Lipid emulsions enriched with fish oil reduced the lymphocytes' capacity to proliferate when stimulated by antigen presentation [SO/FO vs. Control (p = 0.03) and MCT/SO/FO vs Control and MCT/SO (p = 0.001)] (fig. 1).
Effect of different LE on cytokine production
All groups incubated with parenteral lipid emulsions reduced the production of IL-2 compared to the control group (no lipid emulsion). The MCT/SO group presented the lowest reduction of IL-2, whereas the SMOF group presented the highest inhibition rate of cytokine production (SMOF vs. SO, SO/FO, MCT/SO and Control group. p < 0.01) (fig. 2).
The SO group increased the production of IL-6 in contrast to the control and all the groups treated with LEs (p < 0.001). The lowest production of IL-6 was observed in the MCT/SO/FO group (p < 0.001) (fig. 2).
Production of the anti-inflammatory cytokine IL-10 decreased in the SO group when compared to the other groups (p < 0.05). However, the production of IL-10 increased in the SO/FO and MCT/SO/FO groups compared to the control group (p < 0.05) and to groups with no fish oil added: SO and MCT/SO (p < 0.05) as well as to the SMOF group (fig. 2).
Discussion
In the current study we observed an increased incorporation of PUFA, particularly from the n-3 series, at the membrane of monocytes/macrophages and activated lymphocytes when they were cultured with LEs containing fish oil (SO/FO, MCT/SO/FO and SMOF groups). We also found a decrease in n-9 MUFA (oleic acid). Our finding agrees to previous studies where mitogen-activated mononuclear cells cultured with PUFA increased PUFA incorporation at the cell membrane and reduced the concentration of saturated and monounsaturated fatty acids.23,24
The increased incorporation of polyunsaturated fatty acids (PUFA) at the cell membrane enhances its fluidity and consequently the expression of surface molecules of immunological cells. On the other hand, the decrease in PUFA at the membrane and the increase in MUFA and saturated fatty acids decrease membrane fluidity, which can decrease the expression of surface molecules.20-22 Keratinocytes cultured with EPA and arachidonic acid (AA) enhanced the expression of ICAM-1. This finding was associated to the increased fluidity of the cell membrane of keratinocytes after the incorporation of n-3 and n-6 PUFA.22
Theoretically, the change of fatty acid profile, at the cell membrane observed in the current study, should favor the expression of surface molecules. However, the response of surface molecules expression in this study was not consistent to former findings with regard to the lipids emulsions they have been exposed to.
In the present observation, all parenteral lipid emulsions decreased the expression of HLA-DR molecules on activated monocytes/macrophages surface. Regarding containing-fish oil LE groups, and consistent with our findings, Hughes et al. found that activation of human mononuclear cells by INF-gamma and subsequent incubation with EPA and DHA, (using the same ratio naturally found in fish oil), reduced the expression of surface molecules - HLA-DR, HLA-DP and HLA- DQ. They also found a reduction in lymphocyte proliferation after antigen stimulation with the tetanus toxoid antigen.25,26
In the present in vitro study, there was an increase in lymphocyte receptors for the co-stimulatory molecules CD28 and CD152 by all studied lipid emulsions. Regarding containing-fish oil groups, Sasaki et al. had also found enhanced expression of CD28 molecules on the surface of T-lymphocytes in isogenic C57BL/6 mice fed with an oral diet supplemented with n-3 PUFA.21 In the other hand, mice fed with EPA and DHA did not change the expression of CD28, but EPA enhanced the expression of CD152 compared to control animals fed with corn oil, rich in n-6 PUFA. The authors found a decrease in the proliferation of lymphocytes stimulated by antibodies anti-CD3 and anti- CD28 with EPA, suggesting that EPA could inhibit the proliferation of lymphocytes by enhancing the molecule that downregulates the co-stimulatory signal (CD152).27
In the present study, reduced lymphocyte proliferation could be expected for all groups incubated with LEs due to the increased expression of CD152 and reduced production of IL-2, a cytokine witch stimulate lymphocyte proliferation.28,29 However, there was a decreased lymphocyte proliferation only in the groups that received fish oil-based lipid emulsions (SO/FO and MCT/SO/FO). In addition, although SMOF contain fish oil group and had a lower production of IL-2, this parenteral lipid emulsion did not significantly decrease lymphocyte proliferation, indicating a favorable effect of this fish oil-containing lipid emulsion on this variable.
Potential mechanisms that can be involved in the fish oil-related modulation of lymphocyte proliferation include inhibition of cell cycle progression and induction of apoptosis. Jurkat cells incubated with EPA and DHA had reduced lymphocyte proliferation and also inhibited the MAP-kinase signaling pathway, which plays a key role in the progression of the cell cycle during lymphocyte proliferation.30,31 In another in vitro study, EPA and DHA increased the number of lymphocytes in the G0/G1 phase and reduced the ratio of these cells at phases S and G2/M of the cell cycle, confirming that reduced lymphocyte proliferation could be due to inhibition of cell cycle progression.32 In addition, an in vitro study has shown that there is a relationship between the inhibition of lymphocyte proliferation and the pro-apoptotic effect of n-3 PUFA. Incubation with DHA reduced the proliferation of HL-60 cells.33
In vitro studies with lipid emulsion added to the culture media have shown that mechanisms that trigger apoptosis can be activated by the deposit of free radicals in the cell since PUFA are more susceptible to lipid peroxidation.34 The increase in the concentration of free radicals in cell cultures can induce death and affect the cell cytoskeletal structure.35
This latest observation allows us to speculate that in our study the potential inhibitory effect of lymphocyte proliferation found in the experimental mixtures with LEs containing fish oil, except for the new SMOF emulsion, could have been associated with the increased oxidative stress. Although the SMOF group contains fish oil, it is worth emphasizing that this parenteral lipid emulsion has the highest antioxidant content (0.2 g/L) compared to the other groups (approximately 0.1 g/L).
In our study, the experimental mixture of MCT/SO with fish oil emulsion (4:1) had promoted an antiinflammatory effect, with downregulation of the proinflammatory cytokine IL-6 and upregulation of the anti-inflammatory IL-10. Additionally, the experimental mixture of SO with fish oil (4:1) also increased IL-10.
Regarding the differences between in vitro and in vivo studies, especially in studies involving cytokines, our findings also agrees with previous scientific reports of n-3 PUFA which have shown, after an inflammatory stimulus, a decreased production of pro-inflammatory cytokines such as IL-1-alfa and beta, IL-6 and TNFalpha and beta.36-38
During parenteral supply of fish oil, n-3 PUFA can become quickly available to organs, tissues and immune system cells, expediting its modulating effect. In this sense, the infusion of fish-oil based lipid emulsion in healthy volunteers for a short period of time (48 hours) significantly increased the ratio of n-3/n-6 fatty acids at the plasma and at the monocyte membrane and reduced the production of pro-inflammatory cytokines, TNF-alpha, IL-1, IL-6 and IL-8, after stimulation of monocytes with endotoxin. This effect was not observed in the control group, which received the infusion of soybean oil-based LE.39 Clinically, the preoperative parenteral infusion of fish oil-based lipid emulsion in surgical patients also reduced the amount of IL-6.40
In our in vitro study, the soybean oil-based lipid emulsion, rich in n-6 PUFA, increased the pro-inflammatory cytokine IL-6 and reduced the anti-inflammatory cytokine IL-10 in the supernatant of cell cultures. The inflammatory effect of a lipid emulsion rich in n-6 PUFA was also observed in animals with burns, fed with central parenteral nutrition, in which the sunflower oil infusion increased the plasma levels of IL-6 compared to control animals that received no fat in their parenteral nutrition.41 Clinically, the parenteral infusion of the lipid emulsion containing soybean oil in severely stressed surgical patients resulted in increased serum IL-6 compared to the control group that received parenteral nutrition without soybean oil.42
It is interesting to observe that SMOF had a neutral effect on the production of IL-6 and IL-10, although it contains fish oil. Considering that the mixture of MCT/SO with fish oil reduced the level of IL-6 and amplified IL-10, the neutral effect of SMOF could be attributed to the amount of olive oil (25%), rich in n-9 monounsaturated fatty acids. The n-3, n-6 PUFA and n-9 MUFA are incorporated by the cell membranes, but their affinity decreases in the order mentioned.43 We could presume that the increased availability of n-9 affected the incorporation of the n-3 and n-6 series, because the ratio n-6:n-3 is higher at the membrane of monocytes/macrophages and lymphocytes from the SMOF group compared to the MCT/SO/FO group (SMOF = 7:1.4 and MCT/SO/FO = 3.8:1 at the membrane of monocytes/macrophages and SMOF = 4:1 and MCT/SO/FO= 2:1 at the membrane of lymphocytes).
The reduced production of pro-inflammatory cytokines IL-2 and IL-6 and the increase of anti-inflammatory cytokine IL-10 observed with fish oil LE could be of clinical importance as it can modulate the inflammatory response and reduce its severity.
Conclusions
Based on the findings of our study with activated human monocytes/macrophages and lymphocytes, we can conclude that parenteral lipid emulsions, regardless of their fatty acid composition, are responsible for similar modulation of the expression of surface molecules involved in the process of antigen presentation, although this modulation varies depending on the type of cells studied. Adding 20% of fish oil emulsion to convention lipid emulsions containing soybean oil and MCT/soybean oil results in anti-inflammatory effect related to cytokine production and decreased lymphocyte proliferation. The new lipid emulsion -SMOF- containing a mixture of soybean, olive and fish oil, plus medium-chain triglycerides, had a neutral impact on the studied immune and inflammatory variables.
References
1. Lacetera N, Scalia D, Mashek DG, Bernabucci U, Grummer RR. Effects of intravenous triacylglycerol emulsions on lymphocyte responses to mitogens in fasted dairy cows undergoing intense lipomobilization. J Dairy Res 2007; 74: 323-8. [ Links ]
2. De Pablo MA & de Cienfuegos A. Modulatory effects of dietary lipids on immune system functions. Immunol Cell Biol 2000; 78: 31-39. [ Links ]
3. Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr 2002; 56: S14- S19. [ Links ]
4. Campos FG, Waitzberg DL, Habr-Gama A, Logullo AF, Noronha IL, Jancar S, Torrinhas RS, Furst P. Impact of parenteral n-3 fatty acids on experimental acute colitis. Br J Nutr 2002; 87: S83-8. [ Links ]
5. Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr 2006; 30: 351-67. [ Links ]
6. Waitzberg DL, Bellinati-Pires R, Yamaguchi N, Massili-Oku S, Salgado MM, Hypolito IP, Soares SC, Goncalves EL, Furst P. Influence of medium-chain triglyceride-based lipid emulsion on rat polymorphonuclear cell functions. Nutrition 1996; 12: 93-9. [ Links ]
7. Waitzberg DL, Lotierzo PH, Logullo AF, Torrinhas RS, Pereira CC, Meier R. Parenteral lipid emulsions and phagocytic systems. Br J Nutr 2002; 87: S49-57. [ Links ]
8. Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev 2003; 192: 161-80. [ Links ]
9. Granucci F, Zanoni I, Feau S, Ricciardi-Castagnoli P. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J 2003; 22: 2546-51. [ Links ]
10. Grose RH, Howarth GS, Xian CJ, Hohmann AW. Expression of B7 costimulatory molecules by cells infiltrating the colon in experimental colitis induced by oral dextran sulfate sodium in the mouse. J Gastroenterol Hepatol 2001; 16: 1228-34. [ Links ]
11. Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998; 18: 389-418. [ Links ]
12. Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res 1999; 19 (1): 1-24. [ Links ]
13. McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol 1999; 77: 1-10. [ Links ]
14. Vandenborre K, Van Gool SW, Kasran A, Ceuppens JL, Boogaerts MA, Vandenberghe P. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 1999; 98 (3): 413-21. [ Links ]
15. Grimm H, Mertes N, Goeters C et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr 2006; 45: 55-60. [ Links ]
16. Heller AR, Rössler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med 2006; 34: 972-9. [ Links ]
17. Wichmann NW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effect of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 2007; 35: 700-6. [ Links ]
18. Brill A, Baram D, Sela U, Salamon P, Mekori YA, Hershkoviz R. Induction of mast cell interactions with blood vessel wall components by direct contact with intact T cells or T cell membranes in vitro. Clin Exp Allergy 2004; 34: 1725-31. [ Links ]
19. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497-509. [ Links ]
20. Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J 1994; 300: 509-18. [ Links ]
21. Sasaki T, Kanke Y, Kudoh K, Misawa Y, Shimizu J, Takita T. Effects of dietary docosahexaenoic acid on surface molecules involved in T cell proliferation. Biochim Biophys Acta 1999; 1436: 519-30. [ Links ]
22. Lu L, Okada N, Nakatani S, Yoshikawa K. Eicosapentaenoic acid-induced changes in membrane fluidity and cell adhesion molecules in cultured human keratinocytes. Br J Dermatol 1995; 133: 217-22. [ Links ]
23. Ferber E, De Pasquale GG, Resch K. Phospholipid metabolism of stimulated lymphocytes. Composition of phospholipid fatty acids. Biochim Biophys Acta 1975; 398 (3): 364-76. [ Links ]
24. Rode HN, Szamel M, Schneider S, Resch K. Phospholipid metabolism of stimulated lymphocytes. Preferential incorporation of polyunsaturated fatty acids into plasma membrane phospholipid upon stimulation with concanavalin A. Biochim Biophys Acta 1982; 688: 66-74. [ Links ]
25. Hughes DA, Pinder AC. n-3 polyunsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am J Clin Nutr 2000; 71: 357S-60S. [ Links ]
26. Hughes DA, Pinder AC. N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin Exp Immunol 1997; 110: 516-23. [ Links ]
27. Ly LH, Smith R, Switzer KC, Chapkin RS, McMurray DN. Dietary eicosapentaenoic acid modulates CTLA-4 expression in murine CD4+ T-cells. Prostaglandins Leukot. Essent. Fatty Acids 2006; 74: 29-37. [ Links ]
28. Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol 2004; 172: 3983-8. [ Links ]
29. Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6: 595-601. [ Links ]
30. Denys A, Hichami A, Khan NA. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase (ERK1/ERK2) signaling in human T cells. J Lipid Res 2001; 42: 2015-20. [ Links ]
31. Denys A, Hichami A, Khan NA. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase enzyme activity in human T-cells. Mol Cell Biochem 2002; 232: 143-8. [ Links ]
32. Gorjão R, Cury-Boaventura MF, de Lima TM, Curi R. Regulation of human lymphocyte proliferation by fatty acids. Cell Biochem Funct 2007; 25: 305-15. [ Links ]
33. Lawrence C, Chiu M, Elaine Y, Wong L, Vicent EC. Docosahexaenoic acid modulates different genes in cell cycle and apoptosis to control growth of human leukemia HL-60 cells. Int J Oncol 2004; 25: 737-44. [ Links ]
34. Muhlebach SF, Steger PJ. Lipid peroxidation of intravenous fat emulsions: a pharmaceutical issue with clinical impact? Nutrition 1998; 14: 720-1. [ Links ]
35. Bellomo G, Mirabelli F. Oxidative stress injury studied in isolated intact cells. Mol Toxicol 1987; 1 (4): 281-93. [ Links ]
36. Endres S, Ghorbani R, Kelley VE et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of IL-1 and TNF alpha by mononuclear cells. N Engl J Med 1989; 320: 226-71. [ Links ]
37. Endres S, Meydani S N, Dinarello C A. Effects of omega 3 fatty acid supplements on ex vivo synthesis of cytokines in human volunteers. Comparison with oral aspirin and ibuprofen. World Rev Nutr Diet 1991; 66: 401-6. [ Links ]
38. Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996; 63: 116-22. [ Links ]
39. Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W. Short-Time Infusion of Fish Oil-Based Lipid Emulsions, Approved for Parenteral Nutrition, Reduces Monocyte Proinflammatory Cytokine Generation and Adhesive Interaction with Endothelium in Humans. The Journal of Immunology 2003; 171: 4837-43. [ Links ]
40. Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr 2002; 87: S89-S94. [ Links ]
41. Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N, Ito I. Effects of intravenous omega-3 and omega-6 fat emulsion on cytokine production and delayed type hypersensitivity in burned rats receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr 1998; 22 (6): 363-7. [ Links ]
42. Furukawa K, Yamamori H, Takagi K, Hayashi N, Suzuki R, Nakajima N, Tashiro T. Influences of soybean oil emulsion on stress response and cell-mediated immune function in moderately or severely stressed patients. Nutrition 2002; 18: 235-40. [ Links ]
43. Chan S, McCowen KC, Bistrian B. Medium-chain triglyceride and n-3 polyunsaturated fatty acid-containing emulsions in intravenous nutrition. Curr Opin Clin Nutr Metab Care 1998; 1: 163-9. [ Links ]
![]() Correspondence:
Correspondence:
Thiago Manzoni Jacintho.
Av. Dr. Arnaldo, 455, sala 2208.
CEP 01236-903, São Paulo-SP, Brasil.
E-mail: metanutri@terr.com.br; metanutri@usp.br
Recibido: 3-IX-2008.
Aceptado: 2-III-2009.