BACKGROUND
About 8-12 % of couples of childbearing age suffer from infertility (1), which has a serious negative impact on women's physical and mental health and family happiness, so it is important to find an effective way to prevent and treat infertility. unfortunately, the causes of infertility are extremely complex, including ovulation dysfunction (2), tubal disease (3), endometriosis (4), and immune disease (5), and up to 20-30 % of cases have an unknown etiology (6). While older age and previous miscarriages are important factors in infertility, there is a growing consensus that other factors, including lifestyle and environmental factors, play a growing role in female fertility (7-9). Lifestyle and diet intervention management of infertility may be a promising and invaluable strategy.
Researches for the association between dietary factors and infertility have mainly focused on the dietary fatty acid, carbohydrate, lipid, protein, and food related components, such as endocrine disruptors, thinking that the quantity and QUALITY of imbalance between various nutrients may damage metabolism and fertility in women (10). Studies have shown that Mediterranean diet can effectively improve insulin resistance, metabolic disorders and obesity, which are critical for fertility (10,11). Mediterranean diet is characterized by a high intake of vegetables (including legumes), fruit, olive oil, unrefined carbohydrates, and so on (12). As an important component of vegetables, fruits and grains, dietary fiber is reasonably suspected to be associated with fertility. In fact, the average American woman of childbearing age consumes far less dietary fiber (13.8 grams per day) than the recommended amount (20 to 35 grams per day) (13-15). unfortunately, the relationship between dietary fiber and infertility has yet to be established. After summarizing two prospective cohort studies about the association between dietary factors and infertility, Sydney K. Willis found that increasing dietary fiber consumption could result in higher pregnancy rates in women with BMI ≥ 25 kg/m2 (16). However, the studies by Audrey J. Gaskins et al. found that high dietary fiber consumption was significantly associated with lower concentrations of reproductive hormones and an increased risk of anovulation events (17). However, there is a bias there because the method to detect ovulation was by measuring serum progesterone rather than by B-mode ultrasound. In addition, limited researches have studied the nonlinear relationship between dietary fiber and infertility. Therefore, we aimed to explore the linear and non-linear relationship between dietary fiber and infertility through a secondary analysis of a large sample based on the NHANES database from 2013 to 2018.
MATERIALS AND METHODS
STUDY SAMPLES
Data from the National Health and NUTRITION Examination Survey (NHANES) 2013-2018 were used to investigate the association between dietary fiber and infertility. Since the early 1970s, NHANES has been collecting comprehensive data on diet and disease to inform NUTRITION and health policies, and uses a complex sampling design to provide health and NUTRITION information every 2 years from a representative sample of the civilian and non-institutionalized population in the u.S. (18). Informed consent was signed by all participants over the age of 18.
In this research we used data from three consecutive cycles of NHANES (2013-2018), as only three cycles contained the reproductive health questionnaires addressing our issues. The NHANES made available the demographic, dietary, examination, laboratory, and questionnaire-related data, which could be downloaded for free from the official NHANES website (https://www.cdc.gov/nchs/NHANES/index.htm).
VARIABLES
The dependent variable in this study is self-reported infertility obtained from a reproductive health questionnaire. Participants were asked: “Have you ever attempted to become pregnant over a period of at least one year without becoming pregnant?” A “yes” answer was considered as “ever infertile”, and a “no” was classed as “fertile”. People who did not answer the above question were excluded from the research (19).
The independent variable of this study is dietary fiber intake, and the Automated Multiple Pass Method (AMPM) was used, which has been shown to measure group energy intake accurately (20). Participants were required to answer questions about the food and drinks they had consumed in the 24 hours prior to the questionnaire. A variety of measurement guides were used, such as cups and bowls, to ensure a correct calculation of the diet. The detailed process of how to obtain and measure dietary fiber intake is available at the NHANES official website.
Based on previous literature (21-24), the following covariates were included in the analysis, including important demographic variables: age, body mass index (BMI, kg/m2), race, ratio of family income to poverty, education level, poverty income ratio (PIR), marital status, and parity. In addition, unhealthy habits such as drinking and smoking were also analyzed.
STATISTICAL ANALYSIS
The statistical analysis was performed according to the Centers for Disease Control and Prevention (CDC) guidelines (https://wwwn.cdc.gov/nchs/NHANES/tutorials/default.aspx). Because NHANES uses a complex sampling design, we used the weighting methodology in the analysis. Since dietary fiber intake in the population is skewed, a Log10 transform was performed. Mean ± standard deviation, median (quartile) and frequency percentage were respectively used to represent continuous and categorical variables. To estimate the differences between groups in dietary fiber (quartiles), we calculated chi-square tests for categorical variables and weighted linear regression models for continuous variables. To investigate whether dietary fiber consumption is associated with infertility, we performed the following statistical analyses: first of all, we used a multivariate logistic regression to explore the association between log10 dietary fiber and infertility. Three models were used: an unadjusted model, which did not adjust for any covariate; a minimally adjusted model where only age, race, and marital were adjusted; and a fully adjusted model where all covariables were adjusted.
Then, since regression analysis cannot impede nonlinearity, we employed several methods to address the nonlinear association between log10 dietary fiber and infertility. Firstly, we divided dietary fiber into four groups and observed whether the odds ratio of each group had equidistant changes. Secondly, the smooth curve fitting and weighted generalized additive model (GAM) were used to explore whether there was a curvilinear association between dietary fiber consumption and infertility. In addition to binary logistic regression analysis, we also used two-piecewise linear regression and observed whether the two models differed according to the outcome of the log likelihood ratio.
Because some females in the fertile group perhaps never tried to conceive, we performed two subgroup analyses to validate the robustness of the result, including women who had ever given birth and women aged between 35 and 45 years (25).
The statistical analysis was performed using R (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
RESULTS
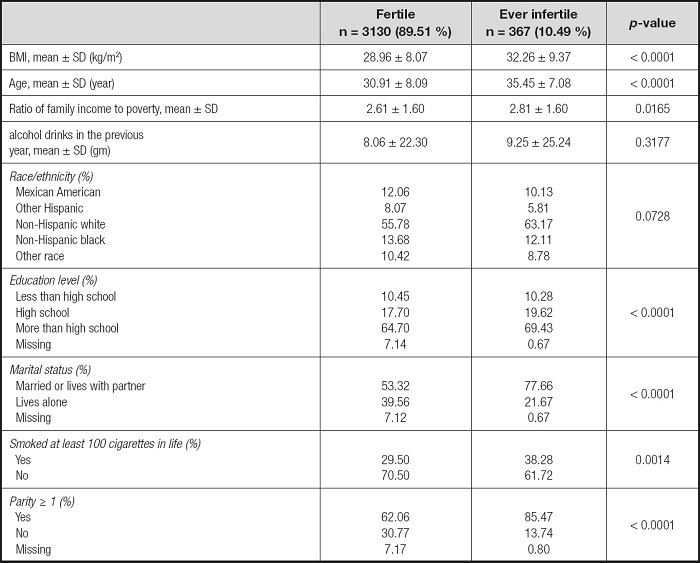
POPULATION CHARACTERISTICS
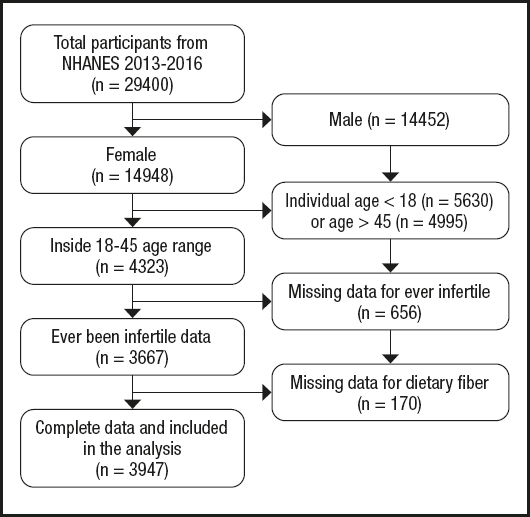
Data from the NHANES database for 2013 to 2018 (3 cycles: 2013-2014, 2015-2016, 2017-2018) were analyzed. Initially, 29,400 civilians during 2013-2018 were involved. Then we excluded participants according to the following criteria: male (n = 14,452), females younger than 18 years (n = 5,630) or aged over 45 years (n = 4,995) (26), females with missing data for infertility (n = 656) and dietary fiber (n = 170). As a result, 3,497 participants were included in our analysis (Table I and Fig. 1).
ASSOCIATION BETWEEN DIETARY FIBER CONSUMPTION AND INFERTILITY
The association between dietary fiber consumption and infertility is shown in table II. In the unadjusted model, a negative correlation between dietary fiber intake and infertility was found (OR = 0.68, 95 % CI: 0.48 to 0.96). Then, we adjusted for age, race and marital status in the minimally adjusted model, and the beneficial effects of dietary fiber intake remained (OR = 0.58, 95 % CI: 0.40 to 0.83). In the fully-adjusted model all covariables were adjusted, and the correlation remained strong (OR = 0.60, 95 % CI: 0.41 to 0.88).
Table II. Univariate and multivariate analysis for the linear and non-linear relationship between dietary fiber and infertility, with subgroups.
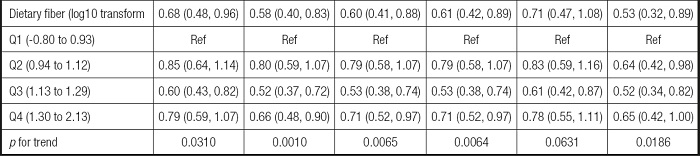
Unadjusted model: no covariates were adjusted. Minimally adjusted model: only adjusted for age, race, marital status. Fully adjusted model: all covariates were adjusted. OR: odds ratio; 95 % CI: 95 % confidence interval; GAM: generalized additive model, continuous covariates were adjusted as non-linearity.
*Covariates adjusted in the two subgroups were same as GAM.
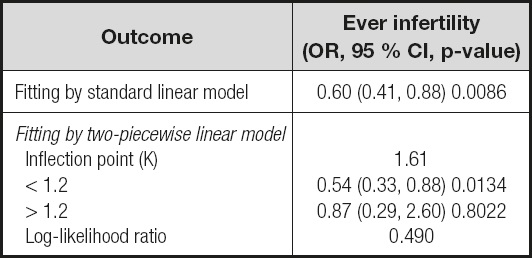
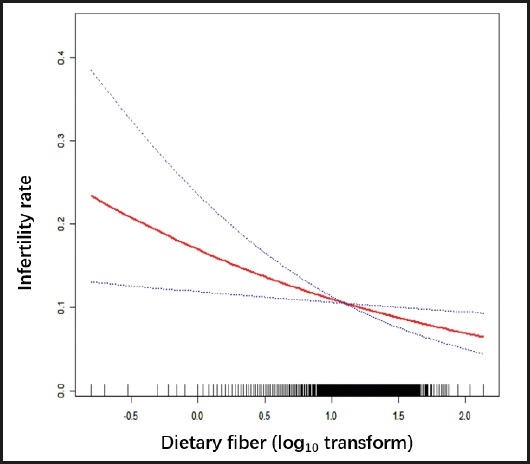
The robustness of the results of the fully adjusted model can be verified by the GAM model as well as p for trend. Firstly, the OR (odds ratio) and CI (confidence interval) of the GAM model was similar to the fully adjusted model. Secondly, the outcome of p for trend was In line with dietary fiber intake when it was analyzed as a continuous variable. But the ORs in different dietary fiber consumption groups were non-equidistant (ref. in Q1; 0.79 in Q2; 0.53 in Q3 and 0.71 in Q4), suggesting that we should consider the possibility of a non-linear association between dietary fiber intake and infertility (Table II). So we further performed a smooth curve fitting (penalty spline method) and a linear relationship was found between dietary fiber intake and infertility (Fig. 2). The RESULTS were also supported by a two-piecewise linear regression (Table III).
According to the previous literature (27), a further subgroup analysis was performed to reveal the potential relationship between dietary fiber and infertility. We divided those who had given birth and those aged 35-45 into two groups. The outcome was still steady in the two subgroups with the RESULTS of those who had given birth (OR = 0.71; 95 % CI: 0.47-1.08) and of women aged 35-45 years (OR = 0.53; 95 % CI: 0.32-0.89) (Table II).
In summary, the association between intake of dietary fiber and infertility is linear, and the two are negatively associated. More dietary fiber consumption might reduce the risk of infertility.
DISCUSSION
Our results suggest that higher dietary fiber consumption was usually associated with a reduced rate of infertility after adjustment for confounding factors. The results obtained from the NHANES support our hypothesis that more dietary fiber intake can reduce the risk of infertility. unfortunately, researches for the relationship between dietary fiber consumption and infertility are limited. Having studied the current literature, we hypothesized that there may be several possible mechanisms by which high dietary fiber intake results in a reduced rate of infertility.
Firstly, high dietary fiber intake improves infertility outcomes through its potential impact on the vaginal microbiota. It is well known that the normal vaginal microbiome is dominated by lactic acid bacteria, and this particular female vaginal microbiome plays an important role in determining many aspects of reproductive health. A meta-analysis has shown a statistical association between vaginal microbiome and infertility (28). Disturbances in vaginal microbiota can lead to bacterial vaginosis, which can induce tubal infertility (25). Previous observational and interventional studies have shown that fiber intake is inversely associated with bacterial vaginosis, regulating the microbiome, enabling Lactobacillus species to dominate and improving cervical and vaginal health (29), and data from an in vitro study further confirmed the possibility of this relationship (30).
Secondly, high dietary fiber consumption improves fertility by enhancing leptin activity and reducing leptin levels (31,32). Leptin can modulate gonadotropins, which can promote ovarian granulocyte and membrane cell function and oocyte maturation. In the pathological state, leptin concentration is elevated, but the effect of leptin is reduced due to leptin resistance (33,34). Dietary fiber can enhance leptin sensitivity through the JAK2 and STAT3 signaling pathway (35,36). One study suggested that higher dietary fiber intake lowers leptin levels and enhances leptin activity, possibly by reducing body weight and development of obesity (37). However, in our study we observed that the association between dietary fiber and lower rates of infertility remained after adjusting for BMI.
Thirdly, high dietary fiber intake can improve ovulation function by regulating insulin resistance, thus improving fertility. Insulin resistance is known to be an important determinant of ovulation function, as high insulin concentrations may up-regulate free testosterone production, leading to hyperandrogenemia (38,39), which is widespread among patients with polycystic ovary syndrome and often leads to ovulation disorder infertility. Several clinical studies (39-43) have shown that improving insulin sensitivity can reduce circulating testosterone, and appears to help improve the outcome of infertile women. Compared to simple carbohydrates, high-fiber foods tend to lower blood sugar, resulting in a lower glycemic Index and improving insulin resistance significantly (44), thus having a beneficial effect on ovulation function.
In addition, the relationship between dietary fiber and infertility can be explained by adiponectin. Multiple studies have shown that high consumption of whole grain cereals can induce higher plasma adiponectin levels (45,46). Adiponectin receptors are found in reproductive tissues such as the ovaries, fallopian tubes, endometrium, and testes (47). In the ovary, adiponectin plays a key role in oocyte maturation, granulocyte cell proliferation, and steroid secretion (48-51). In animal experiments, adiponectin knockout mice had fewer oocytes, more follicular atresia, and a longer secondary ovulation cycle, suggesting that adiponectin played a significant role in follicular development (52). In a retrospective case-control study, higher levels of adiponectin were found in women who became pregnant after IVF and the presence of adiponectin is associated with better fertilization rates and better embryonic development (53,54). This suggests that dietary fiber may improve fertility by increasing adiponectin levels.
Dietary fiber may also be linked to incidence of infertility through oxidative stress. Pathophysiological assessments of infertile couples suggest that oxidative stress may be one of the causes of female infertility (55-58). Several studies have reported a negative correlation between the oxidative status of follicular fluid (FF) and clinical pregnancy rates (59-63). It is currently believed that oxidative stress leads to DNA and cell membrane damage, decreased oocyte quality, and changes in fertilization, and influences embryo quality, implantation and embryo development. The byproducts of dietary fiber fermentation, like sodium butyrate, can increase the activity of antioxidant enzymes such as catalase or heme oxidase-1 (64). Animal studies conducted by Fardet A have shown that diets rich in dietary fiber can improve the redox status of animals (65-67) and reduce oxidative damage. This conclusion is also applicable in humans and has been confirmed by multiple clinical studies (68-70). So we hypothesize that dietary fiber reduces the risk of infertility by reducing oxidative stress.
According to our review, high dietary fiber consumption may reduce the rate of the female infertility, possibly through the following several potential mechanisms, such as regulating vaginal microbiome composition, reducing leptin concentration, increasing insulin sensitivity, and improving oxidative stress and so on. In addition, there may be other potential mechanisms not mentioned in this paper, such as the relationship between dietary fiber and endometrial thickness (71,72).
Based on the results of our study, we argue that higher dietary fiber consumption is associated with a lower incidence of infertility. Due to the changeable of the dietary structure, recognizing the impact of dietary nutrients on women's fertility and guiding women of childbearing age to get the right nutrients from their diet may be a promising and economic way to prevent female infertility and increase pregnancy rates. Our study can provide new insights into the development of human infertility etiology and recommendations for health policy. Our findings identified a new link between dietary fiber consumption and infertility. This conclusion is required to be systematically validated in animal researches and prospective clinical studies, including recommendations on optimal dietary fiber intake to prevent infertility in women of reproductive age, as well as studies on the underlying mechanisms.
This study has some drawbacks. First, due to the inherent limitations of the cross-sectional design, we cannot estimate an exact causal relationship between dietary fiber intake and infertility rates, so the evidence is relatively weak. However, this study provides a new perspective on the causes and prevention of infertility, which could provide a direction for further research on the mechanism. In addition, large prospective cohort studies should be encouraged. Second, there are many metabolites of dietary fiber, so it is impossible to determine which component is useful for our study, and further mechanism research is needed. Third, although we adjusted for several known potential factors for infertility, there were unadjusted confounding factors, such as “number of miscarriages/abortions”, which is considered a risk factor for secondary infertility. However, this information was not provided in the NHANES database and therefore could not be adjusted for, and these confounders could affect the study as all observational studies do.
CONCLUSIONS
Our study found that dietary fiber consumption was independently and negatively correlated with rate of infertility. So women who suffer from infertility should consider too low dietary fiber intake as a potentially harmful factor. We hope the finding of this study can provide beneficial dietary advice for women trying to conceive.