INTRODUCTION
Kidney transplantation (KTx) is the most effective treatment for patients with end-stage renal disease because it increases survival rates, lowers morbidity, improves the quality of life, and reduces healthcare costs (1). Although KTx improves patient prognosis, conditions that occur after KTx may lead to a decreased micronutrient status, including lower vitamin D levels, which increase the risk of graft failure and premature death (2).
Vitamin D deficiency, typically defined as a 25-hydroxyvitamin D [25(OH)D] level of < 20 ng/mL (3), is commonly observed in patients after KTx and can be attributed to several factors, such as decreased food intake, reduced sun exposure, and 1-alpha-hydroxylase deficiency in the kidneys (4). Yin et al. (5) demonstrated that 52 % of recipients had vitamin D deficiency after KTx. The incidence of vitamin D deficiency at 3 and 6 months after KTx has been reported to be 34 % and 23 %, respectively.
A previous study has revealed that vitamin D deficiency is associated with increased albuminuria, which decreases renal function and increases the risk of graft loss (6). In a recent meta-analysis, patients with vitamin D deficiency had an 82 % greater chance of acute rejection than those without vitamin D deficiency, suggesting that an adequate vitamin D status a few days before and after KTx can reduce the chance of acute rejection.
Low 25(OH)D levels cause changes in the renin-angiotensin-aldosterone system, leading to increased blood pressure, modulation of the immune response, and loss of the podocytes, the components of the glomerular filtration barrier (8,9). Therefore, maintaining 25(OH)D levels > 30 ng/mL is beneficial in this population (3). A longitudinal study evaluated albuminuria in 230 patients after KTx according to their vitamin D status and revealed increased albuminuria in patients with lower vitamin D levels (10).
Although the clinical relevance of vitamin D status in renal graft function has been demonstrated, more studies are needed to clarify the time after KTx at which low vitamin D levels may be associated with renal function decline. Therefore, this longitudinal study aimed to evaluate the hypothesis that vitamin D status could be associated with biomarkers of renal graft function in patients at baseline and at 3 and 6 months after KTx.
METHODS
STUDY DESIGN AND PATIENTS
This longitudinal study was conducted at the Nephrology Outpatient Clinic of Onofre Lopes Hospital of the Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil, between August 2015 and January 2017. Patients of both sexes aged > 20 years who had undergone KTx were included. The exclusion criteria were as follows: patients administered vitamin D supplements, patients diagnosed with focal segmental glomerulosclerosis, patients with diabetes mellitus or diabetic kidney disease, cases in which cytomegalovirus infection occurred in the donor or recipient, patients with residual diuresis after KTx, and patients who experienced humoral or cell rejection.
Among the 156 recipients who underwent KTx, 45 met the eligibility criteria and agreed to participate. After initiating the study, one patient died, one patient dropped out, and two patients experienced graft loss; thus, the remaining 42 patients were analyzed.
As immunosuppressive therapeutic regimens, patients were administered mycophenolate mofetil, tacrolimus, and prednisone or mammalian target of rapamycin inhibitors, such as everolimus or sirolimus.
All patients were followed up at baseline and at 3 and 6 months after KTx. They were categorized into two groups according to their 25(OH)D levels—patients with 25(OH)D levels > 30 ng/mL and patients with 25(OH)D levels ≤ 30 ng/mL (3). At each follow-up examination, fasting blood and first morning void urine samples were obtained.
The study was conducted in accordance with the guidelines of the Ethics in Research Committee of Federal University of Rio Grande do Norte and complies with the Declaration of Helsinki (protocol number 1.144.405). All patients provided their written informed consent to participate.
DATA COLLECTION
The following information was obtained from the patients' medical records: age, sex, ethnicity, etiology of renal disease, presence of comorbidities, duration of hemodialysis, time of hospitalization after KTx, immunosuppressive therapy, donor type, donor age, donor cause of death, creatinine level of donor, estimated glomerular filtration rate (eGFR) of donor, and cold ischemia time of maintaining the organs before KTx.
Sun exposure was assessed based on an adapted questionnaire proposed by Hanwell et al. (11). A sun exposure score was calculated for healthy adults using a recall questionnaire assessing daily time in the sun (< 5 min, 5-30 min, > 30 min) and skin exposure (face/hands, face/hands and arms, face/hands and legs, and "bathing suit") for 1 week. Skin color was self-classified according to the five categories adopted by the Brazilian Institute of Geography and Statistics (12). Weight and height were measured to calculate body mass index (BMI),which was classified according to the World Health Organization guidelines (13).
BIOCHEMICAL ANALYSIS
Fasting blood samples were collected for biochemical analysis. Serum creatinine levels were measured using the Wiener kit and CMD-800 automatic biochemistry analyzer (Wiener Laboratories, Rosario, Argentina). Parathyroid hormone (PTH) levels were measured using the UniCel® DxI 800 Immunoassay System (Beckman Coulter, Brea, CA, USA). Circulating 25(OH)D levels were measured by chemiluminescence with ABBOTT® kits using the ARCHITECT i2000SR Immunoassay Analyzer (ABBOTT Diagnostics, Lake Bluff, IL, USA). The cutoff points used for 25(OH)D levels were defined by the Endocrine Society (as follows: "adequate" ≥ 20 ng/mL and "deficiency" ≤ 20 ng/mL. In this study, patients were classified according to 25 (OH)D levels > 30 ng/mL or ≤ 30 ng/mL, as recommended by the Brazilian Society of Endocrinology and Metabolism, which suggests that in some clinical situations, such as chronic kidney disease, 25(OH)D levels > 30 ng/mL can be beneficial (3).
eGFR was evaluated using the Chronic Kidney Disease Epidemiology Collaboration equation (14). Urinary albumin/creatinine ratio (ACR) was assessed using the first morning urine samples. Albumin and creatinine levels in the urine were determined using the Wiener kit and CMD-800 automatic biochemistry analyzer. The cut-off points used for albuminuria, as defined by the Kidney Disease: Improving Global Outcomes (15), were as follows: normal-to-mild, < 30 mg/g of creatinine; moderate, 30-300 mg/g of creatinine; and severe, > 300 mg/g of creatinine.
STATISTICAL ANALYSIS
The distribution of variables was analyzed using the Kolmogorov-Smirnov test. The data are presented as the average or median, when appropriate. Differences among continuous variables at different time points, at baseline and at 3 and 6 months after KTx, were analyzed using the Friedman test, followed by the post hoc Wilcoxon signed-rank test with Bonferroni correction. Variables were also analyzed according to the vitamin D status at each collection time. Data with parametric distribution were analyzed using Student's t-test, whereas variables showing a non-parametric distribution were analyzed using the Mann-Whitney test. Correlations were assessed using Pearson's or Spearman's rank test when appropriate. Differences between categorical variables were tested using the χ2 test. Categorical data are presented as frequencies (percentages). Data were analyzed using Statistical Package for the Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA) and Graph Pad Prism, ver- sion 5.0 software (Graph Pad, Inc., San Diego, CA, USA). Significance level was set at p < 0.05.
RESULTS
The mean participant age was 41.8 ± 12.3 years; most participants were male and had mixed skin. Glomerulonephritis has been considered the main cause of chronic kidney disease. More than half of the patients had no comorbidities. The median duration of hemodialysis was 3 (1.8-7) years and that of hospital stay after KTx was 16.5 (11-32) days. Furthermore, the most widely used therapeutic regimen was that with the immunosuppressants mycophenolate mofetil, ta- crolimus, and prednisone. Most donated kidneys were obtained from deceased donors, with a mean age of 38.2 ± 13.9 years. Head trauma was the main cause of death of the donors. The median creatinine level of the donors was 0.9 (0.8-1.5) mg/dL, eGFR was 84.4 ± 40.8 mL/min/1.73 m², and cold ischemia time was 14.7 ± 11.6 h (Table I).
Table I. Characteristics of patients and donors who underwent KTx.
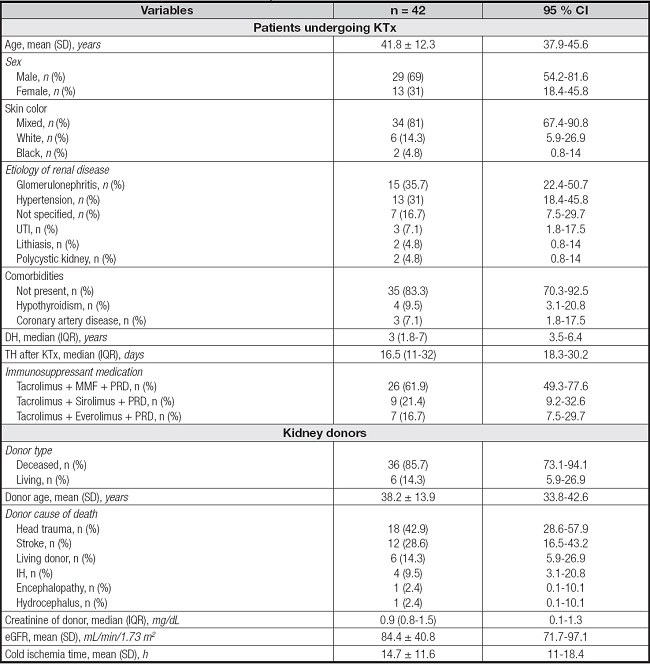
Results are expressed as the mean ± standard deviation or median (interquartile range), except otherwise indicated.
95 % CI: 95 % confidence interval; DH: duration of hemodialysis; eGFR: estimated glomerular filtration rate; IH: intracerebral hemorrhage; IQR: interquartile range; KTx: kidney transplantation; MMF: mycophenolate mofetil; n: number of individuals; PRD: prednisone; SD: standard deviation; TH: time of hospitalization; UTI: urinary tract infection.
When evaluating vitamin D status of patients who underwent KTx, 43% had 25(OH)D levels < 30 ng/mL at baseline. Moreover, 26 % of these patients had 25(OH)D levels < 20 ng/dL during this period, indicating vitamin D deficiency. At the 6-month study follow-up, 38 % patients with 25(OH)D levels < 30 ng/dL at baseline failed to show improvement in their vitamin D status.
There was no significant difference between the BMI values of patients with 25(OH)D levels < 30 ng/mL and those with 25(OH)D levels > 30 ng/mL who underwent KTx. Sun exposure was significantly higher in patients with 25(OH)D levels > 30 ng/dL at 6 months after KTx compared to that in patients with 25(OH)D levels < 30 ng/mL (p = 0.008), which was possibly associated with increased production of vitamin D through exposure of the skin to sunlight. A significant increase in PTH levels was observed at 3 months after KTx(p = 0.008) in patients with 25(OH)D levels < 30 ng/mL compared to that in patients with 25(OH)D levels > 30 ng/mL (Table II), with higher medians than reference values.
Table II. Anthropometric data, sun exposure profile, and biochemical parameters of patients who underwent KTx according to 25(OH)D status considering collection time.
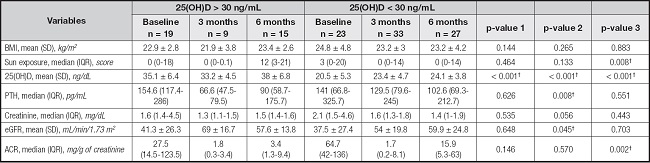
Results are expressed as the mean ± standard deviation or median (interquartile range).
IQR: interquartile range; SD: standard deviation; n: number of individuals; BMI: body mass index; eGFR: estimated glomerular filtration rate; ACR: urinary albumin/creatinine ratio; 5(OH)D: 25-hydroxyvitamin D; PTH: parathyroid hormone. Friedman and Wilcoxon signed-rank tests were used to compare repeated measures with post-hoc Bonferroni correction.
*Significant vs. 3 months (p < 0.008).
Student's t-test or the Mann-Whitney U-test was used to compare groups, as appropriate—p-value 1: baseline [25(OH)D < 30 ng/mL] vs. baseline [25(OH)D > 30 ng/mL]; p-value 2: 3 months [25(OH)D < 30 ng/mL] vs. 3 months [25(OH)D > 30 ng/mL]; p-value 3: 6 months [25(OH)D < 30 ng/mL] vs. 6 months [25(OH)D > 30 ng/mL]—
†Significant p-values.
There was no significant difference between creatinine levels in the different groups; however, the median values at baseline and at 3 months tended to be higher in patients with 25(OH)D levels < 30 ng/mL than in those with 25(OH)D levels < 30 ng/mL. Reflecting this result, eGFR tended to be decreased in patients with 25(OH)D levels < 30 ng/mL at baseline and at 3 months after KTx, although the results were not significant. Twelve (28.6 %) patients with 25(OH)D levels < 20 ng/mL sho- wed no eGFR recovery until 6 months after KTx.
ACR decreased in both groups within 3 months, demonstrating an improvement in post-KTx renal function. However, there was a significant increase at 6 months in patients with 25(OH)D levels < 30 ng/mL compared to that in patients with 25(OH)D levels > 30 ng/mL (p = 0.002; Table II). We found that 44 % patients developed albuminuria after 6 months.
The following variables were associated with 25(OH)D levels. There was a correlation between 25(OH)D levels and ACR (r = -0.444; p = 0.003) at 6 months after KTx, supporting the previous results that demonstrated a worsening of albuminuria during this period in patients with low vitamin D levels. The eGFR correlated with 25(OH)D levels over the 3 months (r = 0.305; p = 0.049), corroborating the comments made in table II that there was a trend toward worse renal function in patients with 25(OH)D levels < 30 ng/mL in this period of the study. Cold ischemia time correlated with 25(OH)D levels at baseline (r = -0.369; p = 0.019) and at 6 months (r = -0.394; p = 0.012).
DISCUSSION
This longitudinal study evaluated vitamin D status in patients who underwent KTx residing in a region of high solar incidence. We found a high frequency of 25(OH)D levels < 30 ng/mL at baseline, which was sustained until the end of the study. The presence of albuminuria at 6 months after KTx was also observed in almost half of the patients who had this vitamin D status profile.
The high percentages of patients with 25(OH)D levels < 30 ng/mL at baseline are consistent with those reported in a previous study, indicating that only 12 % of patients had 25(OH)D levels > 30 ng/mL after KTx. A fact that caught our attention even in our study was that 38 % patients were unable to improve their 25(OH)D status at 6 months (16).
This high frequency of hypovitaminosis D after KTx can be attributed to several factors, such as low sun exposure. Our study demonstrated that patients with 25(OH)D levels > 30 ng/dL had greater sun exposure at 6 months after KTx than those with 25(OH)D levels < 30 ng/mL, which confirms that the incidence of solar radiation increases endogenous vitamin D production.
Although Brazil receives a large amount of solar radiation during the year, specifically in Natal/Rio Grande do Norte, which is a coastal city with the highest solar radiation, there was a considerable frequency of low 25(OH)D levels at 6 months after KTx in our study. These results can be explained by the limitations to sun exposure imposed on this group of patients due to the increased risk of skin tumors (18). Another study conducted in south-eastern Brazil also showed the prevalence of vitamin D deficiency among patients who underwent KTx, even in geographic areas with high exposure to ultraviolet rays (17).
The other factors that may be associated with low vitamin D levels are decreased food intake and 1-alpha-hydroxylase deficiency in the kidneys (4). Progressive loss of the kidney function results in a reduction in the kidney's ability to synthesize active vitamin D. Further, immunosuppressive therapy has been shown to alter vitamin D metabolism, as in the case of the patients in our study. Steroids, for example, express enzymes involved in vitamin D catabolism. Other immunosuppressive agents, such as calcineurin inhibitors, have also been implicated in dysregulation of vitamin D metabolism observed after KTx (19,20).
An important finding of this study is the negative correlation between 25(OH)D levels and albuminuria at 6 months after KTx. During this period, 44 % patients had albuminuria, suggesting the period during which renal function declines. In similar studies, the prevalence of post-KTx albuminuria varied considerably from 7.5 % to 45 % (21). In addition, mild albuminuria (< 500 mg/24 h) at 1 year after KTx was associated with a four-fold increased risk of transplant graft failure (22).
Although this association between vitamin D and renal graft function decline was noted at 6 months, Bienaimé et al. (23) showed that low 25(OH)D levels at 3 months after KTx were associated with worse outcomes in allograft function at 1 year after transplantation. Keyzer et al. (2), in a 7-year follow-up cohort study, also showed that low 25(OH)D levels were significantly associated with decreased renal function and increased mortality. In addition, clinical studies have demonstrated the therapeutic efficacy of vitamin D analogues in reducing proteinuria after KTx (10).
Vitamin D has been shown to protect the kidney by regulating pathways involved in kidney injury, including the renin-angiotensin system and filtration slit diaphragm protein pathways (9,10). In cell culture and mice, Deb et al. (8) demonstrated that podocytes expressing the vitamin D receptor are strongly induced by 1,25-dihydroxyvitamin D3 to stimulate the transcription of nephrin, a key protein in the slit diaphragm synthesized by podocytes. Thus, vitamin D receptor signaling plays a critical role in maintaining the integrity of the slit diaphragm.
Therefore, one of the main extra-osseous effects of vitamin D is represented by its antiproteinuric effect primarily mediated by dysregulation of the renin-angiotensin-aldosterone system. The inverse correlation between calcitriol and renin is an idea consolidated in the literature. Patients with low levels of 25(OH)D (< 30 ng/mL) have been shown to have high levels of renin and angiotensin II due to an inappropriate activation of the renin-angiotensin system (24). These data demonstrate the use of vitamin D due to antiproteinuric function, and it is considered safe in patients with chronic kidney disease and after KTx (25).
This can be explained by the fact that vitamin D exerts potent renoprotective activity that increases graft survival by reducing albuminuria, a major risk factor for renal failure, cardiovascular events, and death (26). Similar to our study, a cross-sectional analysis of the Third National Health and Nutrition Examination Survey database revealed an association between vitamin D deficiency and an increased prevalence of albuminuria in the adult population (27), suggesting that vitamin D has intrinsic antiprotein activity.
The presence of genetic polymorphisms encoding vitamin D binding protein and vitamin D receptor has been recently discussed and is also a factor that may explain the association between vitamin D andrenal function. It is suggested that these polymorphisms can modulate the immune response after allograft transplantation, which can lead to an increased risk of graft rejection and viral infections (28).
The tendency to increase PTH levels in patients with 25(OH)D levels < 30 ng/mL observed in our study corroborates with the findings another study that point out that it is well established that PTH levels are inversely associated with circulating 25(OH)D levels (29). The role of vitamin D is classically associated to the action of PTH on the bone physiology and mineral homeostasis, regulating calcium and phosphate metabolism in different target organs, such as the bones, kidneys, liver, and gastrointestinal tract. Its deficiency often results in bone mineral disorders and development of secondary hyperparathyroidism (30). Thus, studies have demonstrated that vitamin D supplementation can be considered a well-accepted preventive therapy against post-KTx bone loss (31).
In our study, we also observed a negative correlation between 25(OH)D levels and cold ischemia time (Fig. 1). A high cold ischemia time can be associated to the greatest podocyte loss, which is associated with the onset of albuminuria after KTx. This alteration that impairs the integrity of the glomerular filtration membrane (32) and increases blood pressure via negative regulation of the renin-angiotensin system contributes to increased urinary protein excretion. Furthermore, the protective effect of vitamin D as a recognized immunomodulator cannot be offered to recipients with low 25(OH)D levels who underwent KTx in a previous study (9).
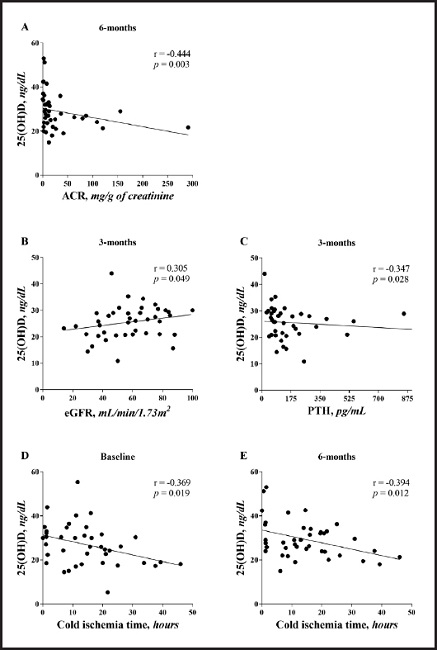
Figure 1. Correlation between 25(OH)D levels, cold ischemia time, estimated glomerular filtration rate, and urinary albumin/creatinine ratio (eGFR: estimated glo- merular filtration rate; ACR: urinary albumin/creatinine ratio).
There are some limitations to our study. It was not possible to obtain food consumption data, thus compromising the association of these parameters with vitamin D status. Thus, large-scale studies on the population who underwent KTx are needed to determine the association between vitamin D levels and renal graft function.
CONCLUSION
Patients frequently have 25(OH)D levels < 30 ng/mL after KTx, even in a region with high sun exposure, with no recovery of this profile within 6 months after KTx in most patients. The biomarkers of renal graft function seem to recover up to 3 months after KTx, but show changes such as the appearance of albuminuria at 6 months, suggesting the period during which renal function declines. These results appear to have a relationship with the vitamin D status of these patients and should be explored in further studies. In the future, we would like to present more comprehensive research results. In addition, randomized clinical trials are needed to assess the effect of vitamin D supplementation on long-term survival outcomes after KTx.














