Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.1 ene. 2007
Use of nonsteroidal antiinflammatory drugs in dental practice. A review
Rafael Poveda Roda 1, José Vicente Bagán2, Yolanda Jiménez Soriano 1, Lola Gallud Romero 4
(1) Staff physician and dentist, Service of Stomatology. Valencia University General Hospital
(2) Professor of Oral Medicine. Valencia University. Chairman of Oral Medicine and Head of the Service of Stomatology. Valencia University General Hospital
(3) Collaborating physician and dentist, Service of Stomatology. Valencia University General Hospital. Valencia (Spain)
ABSTRACT
Nonsteroidal antiinflammatory drugs (NSAIDs) are drugs commonly prescribed in dental practice for the management of pain and swelling. Of these substances, paracetamol and ibuprofen are the most widely used. Their mechanism of action is based on the inhibition of cyclooxygenase, and therefore of prostaglandin synthesis. All of these drugs present a similar mechanism of action, as a result of which their side effects are also similar. The most frequent range from mild (e.g., nausea or vomiting) to serious gastric problems (such as gastric bleeding or perforation). Other side effects include an increased risk of vascular accidents (particularly acute myocardial infarction), renal toxicity secondary to a decrease in perfusion, and the risk of abnormal bleeding tendency due to the antiplatelet effect of these drugs. Their use is contraindicated in the third trimester of pregnancy, due to the induction of premature ductus arteriosus closure. The present study reviews the information currently available on NSAIDs, with special emphasis on those aspects related to dental practice.
Key words: NSAIDs, analgesia, pain, coxibs.
RESUMEN
Los AINES son fármacos de uso común en odontología para el manejo del dolor y de la inflamación siendo paracetamol e ibuprofeno los más utilizados. Su mecanismo de acción está ligado a la inhibición de la ciclooxigenasa y, por tanto, de la síntesis de prostaglandinas. Todos poseen un mecanismo de acción similar por lo que los efectos secundarios también son comunes. Los más frecuentes corresponden a alteraciones gástricas leves (como náuseas o vómitos) o graves (como hemorragia o perforación gástrica). Otros efectos secundarios incluyen el riesgo aumentado de accidentes vasculares (en particular de infarto agudo de miocardio), toxicidad renal por disminución de la perfusión y riesgo de diátesis hemorrágica por el efecto antiagregante plaquetario de estos fármacos. Su uso esta contraindicado en el tercer trimestre del embarazo por inducir el cierre prematuro del conducto arterioso. La intención del presente trabajo es revisar la información actual disponible sobre AINES con especial atención en aquellos aspectos relacionados con la odontoestomatología.
Palabras clave: AINES, analgesia, dolor, coxibs
Introduction
During the year 2004, and through the Spanish National Health Care System (Sistema Nacional de Salud, SNS), nonsteroidal antiinflammatory drugs (NSAIDs) and other antipyretic analgesics (paracetamol) were prescribed for a total of 330.33 million euros – representing 3.34% of the total drug prescriptions made through the SNS. Ibuprofen was the NSAID generating the greatest expenditure, with a total of 16.7 million containers, representing approximately 100.3 million euros. In other words, ibuprofen accounted for a little over 30% of the total NSAID prescription costs. The most extensively prescribed analgesic was paracetamol, with 30.8 million containers, representing a total cost of 86.7 million euros. Paracetamol moreover was the most widely SNS-prescribed drug substance during 2004, followed by omeprazole, with approximately 24.5 million containers, and ibuprofen in third place (1).
In the Valencian Community, and during the same time period, a total of 13 million NSAID and other antipyretic-analgesic containers were prescribed.
The prescription of drugs by dentists and stomatologists through the SNS in the Valencian Community represented 0.079% of the global prescriptions and 0.0417% of the total cost. Analgesics and antiinflammatory drugs for the same period and for the same health care professional sector represented approximately 20,000 prescriptions (20,302), of which 60.88% corresponded to ibuprofen, 14.5% to pyrazolones (metamizol), and 10% to paracetamol. Oxicams (5%) and acetic acid derivatives (5%) practically accounted for the total of prescriptions in this therapeutic group. A significant observation is the fact that only three drugs (ibuprofen, paracetamol and metamizol) accounted for 85% of global analgesics-antiinflammatory drug prescription by dental professionals.
The NSAIDs constitute a heterogeneous group of drugs with analgesic, antipyretic and antiinflammatory properties that rank intermediately between corticoids with antiinflammatory properties on one hand, and major analgesics – opioids on the other.
Self-medication practices are very common with these drugs, and although they are generally quite safe, some have important side effects such as upper gastrointestinal bleeding, endoscopically detectable gastroduodenal ulcers, or an increased risk of vascular accidents such as acute myocardial infarction. Patients therefore must be made aware of the need to use such medication by prescription only.
Classification
Although the NSAIDs show clear differences in biochemical structure and origin, they present a similar mechanism of action; as a result, their side effects are also similar. This situation is known as a "group effect". The differences are to be found in the half-life of each drug, which serves to define the respective dosing intervals; and in the relative potency of each NSAID – this in turn determining the amount of drug substance to be administered in each dose.
Table 1 shows the NSAIDs found on the Spanish market in late 2005. Due to considerations of space, the associations of NSAIDs with other drug substances have been excluded. For each group of NSAIDs, the active drug substance and marketed pharmaceutical formulations are listed, to a maximum of four. Topical formulations have been excluded. The information has been obtained from the Drug Catalog of the year 2006, published by the Spanish General Council of Official Pharmaceutical Colleges (Consejo General de Colegios Oficiales de Farmacéuticos) (2), where the reader may obtain more detailed information.
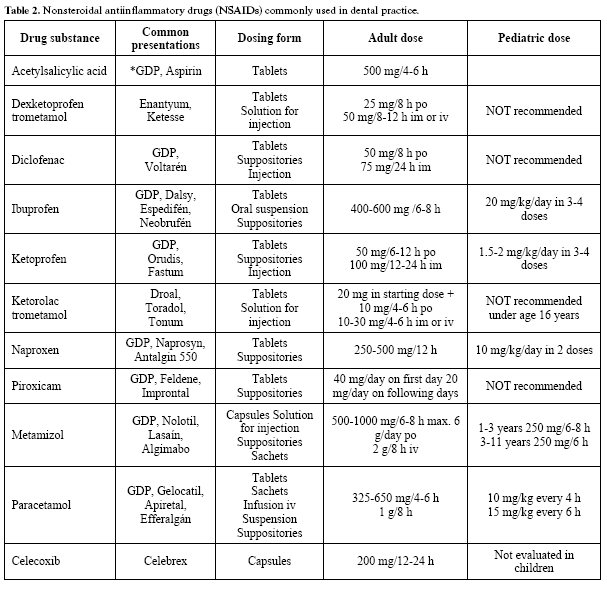
Table 2 in turn reports the most commonly used NSAIDs, specifying the indications, forms of presentation, and the adult and pediatric doses.
General mechanism of action
NSAIDs inhibit cyclooxygenase (COX) – the enzyme responsible for the transformation of arachidonic acid into prostaglandins and thromboxanes, which are substances generically referred to as eicosanoids because their precursors contain 20 carbon atoms. Arachidonic acid is fundamentally found in the cell membrane, where it is bound to phospholipids. Physical, chemical or mechanical stimuli (tissue damage, hypoxia, immune processes, etc.) induce arachidonic acid release and metabolization. The resulting metabolites (prostaglandins and thromboxanes) exert effects on practically all body organs and tissues. In relation to inflammation, the prostaglandins generally exert potent vasodilating action, resulting in increased vascular permeability, with the extravasation of fluids and white blood cells – all these phenomena contributing to inflammation. Consequently, the inhibition of cyclooxygenase synthesis exerts a clear anti-inflammatory effect.
Of particular importance in anti-inflammatory therapy was the identification of two different forms (isoenzymes) of cyclooxygenase: cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2).
COX-1 is implicated in general homeostasis, and is found in most organs and tissues (constitutive isoenzyme). In contrast, COX-2 is not detected in tissues, and only appears in response to certain stimuli (inducible isoenzyme). Based on the hypothesis that selective COX-2 inhibition would induce the desired anti-inflammatory effects without the undesirable side effects (particularly at gastric level) associated with COX-1 inhibition, drugs known as "coxibs" or selective COX-2 inhibitors have been developed.
The analgesic effect of NSAIDs results indirectly from the reduction of inflammation, and also directly due to action upon the central nervous system (CNS).
Analgesic potency. Efficacy
The literature abounds with publications that compare the relative efficacy of one or more NSAIDs in application to a given disease process – either alone or in combination with other drugs. There are also many articles that explore the analgesic efficacy of NSAIDs in the management of swelling and pain secondary to the removal of third molars. No classification has been established of the analgesic-anti-inflammatory efficacy of the different NSAIDs equivalent to that developed for corticoids.
However, the NSAIDs exhibit a "ceiling effect" whereby once the recommended maximum dose has been administered, further dose increments do not elicit an increased analgesic effect. Therefore, the total daily doses of the different NSAIDs offer equivalent efficacy.
In the year 2000, Gøtzsche (3) published a review on nonsteroidal antiinflammatory drugs in which the analysis of a large number of meta-analyses and controlled clinical trials revealed no important differences in effect among the different NSAIDs. The study focused on rheumatoid arthrosis, osteoarthrosis and acute musculoskeletal symptoms. This author likewise found no evidence to suggest that NSAIDs are more effective than paracetamol in application to acute musculoskeletal syndromes. In this sense, Woo et al. (4) in 2005 published a study on the efficacy of NSAIDs and paracetamol in the management of pain following musculoskeletal limb injury, contrasting the efficacy of paracetamol, indomethacin, diclofenac and paracetamol plus diclofenac immediately after trauma and over the subsequent days. Although the combination paracetamol-diclofenac was the first to achieve significant pain relief and induced the greatest reduction in pain (as evaluated by a visual analog scale, VAS), there were no statistically significant differences in mean pain reduction among the different options. The authors concluded that for the dosage, frequency and administration routes used, the differences in analgesic benefit are small and of questionable significance.
Another hypothesis in relation to NSAIDs is the possibility of an increase in effect upon raising the dose. In this sense, and as has been commented above, it seems clear that the NSAIDs present a dose-response relationship that reaches saturation at high doses ("ceiling effect"), in the same way as most other drugs (3).
The preoperative administration of NSAIDs appears to be more effective for the control of pain than postoperative administration in both oral surgery and in general surgery (5,6), though some authors have reported no differences between administration before or after surgery for certain NSAIDs (ketoprofen, diclofenac) (7,8). From the biological perspective, the reduction in prostaglandin production would attenuate the central and peripheral nervous system responses to the adverse stimuli. This reduction in the response to pain could reduce the central and peripheral sensitization phenomena – and thus patient response to subsequent painful stimuli (9,10). However, no differences have been found in the concentration of PGE2 in serum with the pre- and postoperative administration of NSAIDs (ketoprofen) (11).
Although the results of the studies published in the literature are conflicting, it has been suggested that the pharmacological treatment of postoperative pain should begin before surgery and extend to the immediate postoperative period (12).
Side effects
The term "side effects" is to be preferred instead of "adverse effects", since as will be seen below, not all the effects described are adverse.
In general terms, the different NSAIDs have a similar mechanism of action – as a result of which their side effects are also similar.
Gastrointestinal alterations
Gastrointestinal alterations are associated to one degree or other with all NSAIDs. In patients who use these drugs on a routine basis, nausea or dyspepsia is observed in up to 50% of cases, with endoscopically manifest ulcerations in 40% of patients who consume NSAIDs during a period of three months – though most of these lesions have no clinical expression. The symptoms of dyspepsia are not correlated to serious complications (gastrointestinal bleeding or intestinal perforation) (13). The incidence of gastric or duodenal ulceration in chronic treatments is estimated to be 15% and 5%, respectively (14).
The greatest consumption of NSAIDs corresponds to arthrosis-arthritis, typically in elderly patients and with chronic treatment regimens. In dental practice the pattern of use is different: with the exception of some isolated disorder (e.g., chronic disorders of the temporomandibular joint such as osteoarthrosis), NSAIDs are prescribed for short periods of time in patients of all age groups, and in relation to acute infectious-inflammatory processes, or as prophylaxis against such processes. When used in this way, the most frequent side effects are dyspepsia, diarrhea and abdominal pain (15).
It is particularly important for the clinician to know which NSAIDs present the greatest gastrointestinal complication risk. In a meta-analysis conducted by Henry et al. (16) to evaluate the risk of serious gastrointestinal complications (hospital admission due to bleeding or perforation) associated with 12 commonly used NSAIDs, ibuprofen was seen to pose the least risk of gastrointestinal toxicity. In contrast, the relative risk (RR) of some NSAIDs compared with ibuprofen (RR=1) reached values of up to 3.8 (piroxicam) and 4.2 (ketoprofen). The second least deleterious drug in this study was diclofenac. Indomethacin, naproxen, sulindac and acetylsalicylic acid (aspirin) ranked intermediate in this study. The use of higher ibuprofen doses is associated with an increase in gastrointestinal toxicity comparable to that of naproxen or indomethacin.
As to gastrointestinal complications according to the duration of administration, Richy et al. (17) found the maximum relative risk to correspond to the indole derivatives (indomethacin), with a value of 2.25, while the maximum frequency of gastrointestinal complications was recorded after 14 days of treatment. With other NSAIDs the relative risk was clearly lower (naproxen 1.83, diclofenac 1.73, piroxicam 1.66 and ibuprofen 1.19) – the risk being maximum after 50 days.
The risk of important gastrointestinal damage depends on the dose and increases dramatically when more than on NSAID is used (14).
The great therapeutic value of these drugs has made it necessary to develop strategies for avoiding the gastrointestinal risks and allow continued use. Of these strategies, two are of particular interest in our case: the associated administration of gastric mucosa protectors and development of the selective COX-2 inhibiting NSAIDs known as coxibs.
Omeprazole is a proton pump inhibitor (this pump being essential for acid hydrogen ion secretion into the gastric lumen). Its administration at a dose of 20 mg/day affords a 90-95% reduction in 24-hour gastric acid output. Its convenient dosing characteristics; scant, moderate and transient adverse reactions that generally do not require drug withdrawal; and its confirmed efficacy define omeprazole as the drug of choice when gastric protection must be associated to NSAID treatment.
Ranitidine is a specific histamine H-2 receptor antagonist. These receptors are located in the gastric parietal cells, and their blockage by ranitidine inhibits gastric secretion. The drug is administered at a dose of 150 mg every 12 hours, and usually via the oral route. It is well tolerated, and the adverse reactions are scarce.
Misoprostol is an analog of prostaglandin E-1 that binds to the specific prostaglandin receptors located in the gastric parietal cells, giving rise to inhibition of acid production. In addition, it increases the production of mucus and bicarbonate. For the prevention of gastroduodenal ulcer associated to NSAIDs, the recommended dose is 200 µg/6-12 hours. The most common adverse effects are diarrhea, abdominal pain and vomiting.
In the review published by Gotzsche (3), misoprostol is reported to reduce severe gastrointestinal complications and symptomatic ulcers, while the proton pump and H-2 inhibitors reduce endoscopically diagnosed ulcerations – though the clinical relevance of these findings is uncertain.
In another study, Hippisley-Cox et al (18), investigating the adverse effects of NSAIDs, concluded that the administration of gastroprotectors reduces the risk of adverse gastrointestinal events in all NSAID groups except diclofenac.
As has been commented when describing the mechanism of action of the coxibs (selective COX-2 inhibitors), these drugs appeared on the market in response to the idea that selective COX-2 inhibition would reduce or even eliminate the adverse gastric effects of the NSAIDs. The first to be marketed were rofecoxib (withdrawn in September 2004) and celecoxib. Although their principal indication was for chronic joint disease, the Summary of Product Characteristics (SPC) of rofecoxib also specified the treatment of acute pain, and some studies have confirmed its therapeutic efficacy in these processes (19-21)- though the recorded efficacy was no greater than that of the conventional NSAIDs.
It should be stressed that although these drugs are considered to causes fewer gastric complications, large reviews (18) have been unable to draw consistent conclusions regarding their presumed superior gastrointestinal safety versus the non-selective NSAIDs. All of them imply significantly increased risk, except celecoxib. On the other hand, the coxibs can worsen gastrointestinal inflammatory disease; as a result, they are contraindicated in patients with ulcerative colitis and in cases of Crohns disease. They are likewise not without some other adverse effects of NSAIDs such as water retention and kidney damage (as will be discussed below).
A dozen observational studies have been published, revealing a 40-50% reduction in the risk of colon cancer among patients using NSAIDs. This preventive effect is believed to be related to the capacity of these drugs to restore apoptosis and inhibit angiogenesis. However, this beneficial effect is scantly relevant in dental practice, for although the data regarding the amount and duration of NSAID consumption are inconsistent, it seems that risk reduction increases with the frequency of NSAID use (22).
Cardiovascular complications
Although the Summary of Product Characteristics defines acute myocardial infarction as a rare complication of rofecoxib (< 0.01%), the clinical use of the drug was associated with an increase in the registered number of vascular accidents – both acute myocardial infarction and stroke. As a result, the marketing company removed the drug from the market in September 2004. Authors such as Juni et al., in a study published in Lancet in 2004 (23), consider that the drug should have been removed long before, since in the year 2000 clinical trials had already identified relative risks of 2.30, and one year later a RR of 2.24 was recorded for acute myocardial infarction. The European Medicines Evaluation Agency (EMEA), after assessing the cardiovascular risks of this group of drugs (celecoxib, etoricoxib, valdecoxib and paracoxib), considered them to be contraindicated in patients with a history of ischemic heart disease, ischemic stroke, grade II-IV heart failure or peripheral arterial disease.
The Agency also recommends special caution in the use of these drugs when the patients present cardiovascular risk factors (diabetes, hypertension, hyperlipidemia, etc.) (2).
In this sense, an observational study published by Hippisley-Cox and Coupland (24) reported a significantly increased risk of acute myocardial infarction associated with the habitual use of rofecoxib, diclofenac and ibuprofen, with odds ratios (ORs) of 1.32, 1.55 and 1.24, respectively.
Posterior studies have confirmed an increased risk of cardiovascular events with the use of NSAIDs, and even with paracetamol under certain conditions. Chan et al. (25), in a prospective study of 71,979 women during 12 years, found that those who occasionally consumed NSAIDs or paracetamol (1-21 doses/month) were not at an increased risk of cardiovascular accident. Frequent consumption (> 22 doses a month) of NSAIDs presented a relative risk of 1.44, while frequent consumption of paracetamol implied a relative risk of 1.35. The use of 15 or more tablets a week boosted the risk to 1.86 for NSAIDs and 1.68 for paracetamol. Smoking in turn substantially worsened these figures. The authors concluded that the habitual use of NSAIDs or paracetamol implies a modest but statistically significant risk increase for cardiovascular events, while more moderate consumption poses no significant risk. Some studies have obtained clearly different result. Thus, in 2002, Solomon et al. conducted a case-control study in the United States (26) of 4425 patients admitted to hospital due to acute myocardial infarction. Risk in patients who used NSAIDs was seen to be the same as in those who did not. Naproxen was even associated with a significant reduction in the risk of acute myocardial infarction.
Fisher et al. (27) of Basel University, studied the risk of acute myocardial infarction during NSAID treatment and after treatment cessation. They observed a relative risk increment to 1.52 in those subjects that had ceased treatment between 1 and 29 days previously. This risk was not observed in the patients who were consuming NSAIDs at the time of the study or in the patients who had ceased treatment 60 or more days previously. The conclusion of the authors was that the risk of acute myocardial infarction increases during a number of weeks after treatment discontinuation.
In conclusion, most studies reporting an increased risk of infarction associated with NSAIDs do so in patients who consume or have consumed such medication for long periods of time. The studies evaluating only occasional NSAID consumption have revealed no rise in relative risk. Consequently, the use made of these drugs in dental practice can be considered safe from this perspective.
Antiplatelet action
The non-selective inhibition of cyclooxygenase induced by the conventional NSAIDs induces a reduction in thromboxane A2 synthesis, which in turn leads to the inhibition of platelet aggregation – an important step in hemostasia. This is particularly relevant in a specialty with surgical applications such as dental practice.
The incapacity of the platelets to produce cyclooxygenase explains why acetylation of this enzyme induces an irreversible toxic effect that persists for the full life of the platelet (7-10 days) in the case of aspirin, and is reversible in the case of the rest of NSAIDs (14).
Braganza et al. studied gingival bleeding after administering ibuprofen prior to periodontal surgery in 15 patients (28). The same patients in turn served as controls in second surgery in another region of the oral cavity, without prior ibuprofen dosing. In each case bleeding time was determined, together with the papillary bleeding index (after probing the papillary sulcus along the mesial and distal aspects, and classifying bleeding into four grades) and the amount of blood collected during the surgical intervention. The analysis of the results showed that when ibuprofen was administered prior to surgery, a significant increase in intraoperative bleeding was observed – though in neither case did the blood losses have clinical relevance. A significant increase was also seen in bleeding time. However, the papillary bleeding index showed no significant differences according to whether ibuprofen was administered or not.
When contemplating surgery, it is advisable to suppress NSAID medication according to the protocol recommended by the United States Food and Drug Administration (FDA) (15):
4-5 days before the intervention in the case of aspirin
3-4 days before in the case of NSAIDs administered once a day
2-3 days before in the case of those administered 2-3 times a day
1-2 days before in the case of those administered 4-6 times a day
Effects on blood pressure
Another adverse effect of NSAIDs is arterial hypertension (AHT). The consumption of more than 500 mg/day of paracetamol was found to be associated with an increased risk of AHT (RR=1.93 in women between 51-77 years of age, and 1.99 in women between 34-43 years of age), while the consumption of other NSAIDs was associated with relative risks of 1.78 and 1.60 respectively. In the mentioned study (29), the use of aspirin was not associated with an increased risk of AHT – though earlier studies had reported a relationship between arterial hypertension and aspirin (30). The authors concluded that high doses of paracetamol and NSAIDs increase the risk of AHT.
As to the short-term effects of these drugs, the administration of paracetamol at a dose of 4 g/day during one month in hypertensive patients was seen to induce a 4 mmHg rise in systolic pressure (31).
In elderly patients it has been seen that those receiving treatment with NSAIDs in the preceding 60 days have a risk of requiring the introduction of antihypertensive therapy (odds ratio) of 1.66 versus those who have received no such treatment. Moreover, the mentioned risk was seen to increase with the magnitude of the administered daily dose (32).
Since the rises in blood pressure associated to NSAIDs have been found to accumulate in patients with treated arterial hypertension, with only small and nonsignificant increments in normotensive individuals, it has been suggested that NSAIDs could antagonize the effects of antihypertensive drugs. The results published by Johnson et al. support this idea (33).
Kidney toxicity
In individuals with normal kidney function, NSAID mediated acute renal toxicity is practically negligible. In patients with diminished kidney perfusion (hypotension, cirrhosis, congestive heart failure, diuretic use or blood loss), the kidneys increase prostaglandin synthesis to raise the filtration rate and secure an adequate renal flow. However, inhibition of prostaglandin synthesis by NSAIDs can trigger renal hypoperfusion, nephrotic syndrome or interstitial nephritis. The risk is high for indomethacin and phenylbutazone, intermediate for naproxen, ibuprofen, diclofenac, piroxicam and aspirin, and practically inexistent for paracetamol (14).
The most commonly observed side effect is peripheral edema secondary to inhibition of prostaglandin E2 synthesis. The edema and sodium retention are usually moderate, and the result is a 1-2 kg increase in body weight in the first weeks of treatment. In some rare cases the condition may be severe, with marked edema, important weight gain, blood pressure increments and heart failure (34).
Acute kidney failure with rises in blood urea, creatinine and potassium is a rare but serious complication. The condition manifests a few days after starting treatment, and appears faster with short half-life NSAIDs. It has occasionally been reported in apparently healthy individuals (35).
Attention has been drawn to the possibility of chronic interstitial nephropathy and chronic renal failure associated with the use of NSAIDs - including paracetamol.
Use of NSAIDs in pregnancy
The ductus arteriosus diverts an important proportion of pulmonary artery blood flow towards the descending aorta. Under normal conditions the duct functionally seals off immediately after delivery. Koren et al. (36), after reviewing the publications on the use of NSAIDs in the third trimester of pregnancy with premature closure of the ductus arteriosus, found the risk of such premature closure to increase as much as 15-fold in the offspring of women who had used indomethacin for short periods of time. The authors concluded that the use of NSAIDs in the final stages of pregnancy implies a significant increase in the risk of premature closure of the ductus arteriosus. This effect occurs with both selective and non-selective cyclooxygenase inhibitors (37), and is even observed with NSAIDs that are only weak prostaglandin inhibitors – such as nimesulide – (38) despite the fact that premature duct closure and diminished kidney flow in the fetus are fundamentally considered to be a result of the inhibition of prostaglandin synthesis. As was commented at the start of this article, this cannot be regarded as an adverse effect in the strict sense of the word, since the same effect applies when administering NSAIDs as pharmacological treatment for persistent ductus arteriosus.
According to Ostenten et al., the classical, non-selective COX inhibiting NSAIDs do not increase the risk of congenital malformations, though they may be associated with infertility and miscarriage (39). These authors report that treatment with cyclooxygenase inhibitors should be suspended in week 12 of pregnancy in order to avoid the aforementioned problems.
The FDA includes most NSAIDs in category B during the first three months of pregnancy (without evidence of teratogenicity in animals, or with a certain teratogenic potential – though without confirmation in humans). During the last three months of pregnancy, the NSAIDs are included in category D of the FDA (the studies show teratogenic effects, though in some cases the benefits may outweigh the foreseeable risks) (2).
Paracetamol is adequate during pregnancy, though it is advisable to prescribe the drug in consultation with an obstetrician.
Bibliografía
1. Grupos terapéuticos y principios activos de mayor consumo en el SNS durante 2004. Información Terapéutica del Sistema Nacional de Salud 2005;29:49-53. [ Links ]
2. Catálogo de Medicamentos. Colección Consejo Plus 2006. Madrid. Consejo General de Colegios Oficiales de Farmacéuticos 2006.
3. Gøtzsche P. Non-steroidal anti-inflamatory drugs. BMJ. 2000;320:1058-61.
4. Woo WW, Man SY, Lam PK, Rainer TH. Randomized double-blind trial comparing oral paracetamol and oral nonsteroidal antiinflammatory drugs for treating pain after musculoskeletal injury. Ann Emerg Med 2005;46:352-61.
5. Gramke HF, Petry JJ, Durieux ME, Mustaki JP, Vercauteren M, Verheecke G, et al. Sublingual piroxicam for postoperative analgesia: preoperative versus postoperative administration: a randomized, double-blind study. Anesth Analg. 2006;102:755-8.
6. Ong KS, Seymour RA, Yeo JF, Ho KH, Lirk P. The efficacy of preoperative versus postoperative rofecoxib for preventing acute postoperative dental pain: a prospective randomized crossover study using bilateral symmetrical oral surgery. Clin J Pain 2005;21:536-42
7. Kokki H, Salonen A. Comparison of pre- and postoperative administration of ketoprofen for analgesia after tonsillectomy in children. Paediatr Anaesth 2002;12:162-7.
8. Norris A, Un V, Chung F, Thanamayooran S, Sandler A, Katz J. When should diclofenac be given in ambulatory surgery: preoperatively or postoperatively?. J Clin Anesth 2001;13:11-5.
9. Dahl JB, Moiniche S. Pre-emptive analgesia. Br Med Bull 2004;71:13-27.
10. Ochroch EA, Mardini IA, Gottschalk A. What is the role of NSAIDs in pre-emptive analgesia? Drugs 2003;63:2709-23.
11. Wnek W, Zajaczkowska R, Wordliczek J, Dobrogowski J, Korbut R. Influence of pre-operative ketoprofen administration (preemptive analgesia) on analgesic requirement and the level of prostaglandins in the early postoperative period. Pol J Pharmacol 2004;56:547-52.
12. Filos KS, Vagianos CE. Pre-emptive analgesia: how important is it in clinical reality?. Eur Surg Res 1999;31:122-32.
13. Baos V. Los efectos adversos más frecuentes de los 20 principios activos más consumidos en el SNS durante 2000. Información Terapéutica del Sistema Nacional de Salud 2001;25:161-8.
14. Feria M. Fármacos analgésicos, antitérmicos y antiinflamatorios no esteroideos. Antiartríticos. En: Flórez J, Armijo JA, Mediavilla A, eds. Farmacología Humana. Barcelona: Masson; 2005. p. 375-408.
15. Ganzberg S. Analgésicos opiáceos y no opiáceos. En: American Dental Association. Terapéutica dental. Barcelona: Masson; 2003.
16. Henry D, Lim LL, Garcia Rodriguez LA, Perez Gutthann S, Carson JL, Griffin M, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996;312:1563-6
17. Richy F, Bruyere O, Ethgen O, Rabenda V, Bouvenot G, Audran M, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 2004;63:759-66
18. Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;331:1310-6.
19. Chiu WK, Cheung LK. Efficacy of preoperative oral rofecoxib in pain control for third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:47-53.
20. Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction. Br Dent J 2004;197:407-11.
21. Jackson ID, Heidemann BH, Wilson J, Power I, Brown RD. Double-blind, randomized, placebo-controlled trial comparing rofecoxib with dexketoprofen trometamol in surgical dentistry. Br J Anaesth 2004;92:675-80.
22. Sansbury LB, Millikan RC, Schroeder JC, Moorman PG, North KE, Sandler RS. Use of nonsteroidal antiinflammatory drugs and risk of colon cancer in a population-based, case-control study of African Americans and Whites. Am J Epidemiol 2005;162:548-58.
23. Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 2004;364:2021-9.
24. Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005;330:1366.
25. Chan A, Manson J, Albert C, Chae C, Rexrode K, Curhan G, et al. Nonsteroidal antiinflamatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 2006;113:1578-87.
26. Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med 2002;162:1099-104.
27. Fisher LM, Schlienger RG, Matter CM, Jick H, Meier CR. Discontinuation of nonsteroidadl anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med 2004;164:2472-6
28. Braganza A, Bissada N, Hatch C, Ficara A. The effect of non-steroidal anti-inflammatory drugs on bleeding during periodontal surgery. J Periodontol 2005;76:1154-60.
29. Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension 2005;46:500-7.
30. Dedier J, Stampfer MJ, Hankinson SE, Willett WC, Speizer FE, Curhan GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension 2002;40:604-8.
31. Chalmers JP, West MJ, Wing LM, Bune AJ, Graham JR. Effects of indomethacin, sulindac, naproxen, aspirin and paracetamol in treated hypertensive patients. Clin Exp Hypertens A 1984;6:1077-93
32. Gurwitz JH, Avorn J, Bohn RL, Glynn RJ, Monane M, Mogun H. Initiation of antihypertensive treatment during nonsteroidal anti-inflammatory drug therapy. JAMA 1994;272:781-6.
33. Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 1994;121:289-300.
34. Harris R COX-2 and the Kidney J. Cardiovasc pharmacol 2006;47:37-41
35. Brater DC, Harris C, Redfern JS, Gertz BJ. Renal effects of COX-2-selective inhibitors. Am J Nephrol 2001;21:1-15.
36. Koren G, Florescu A, Costei AM, Boskovic R, Moretti ME. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother 2006;40:824-9.
37. Toyoshima K, Takeda A, Imamura S, Nakanishi T, Momma K. Constriction of the ductus arteriosus by selective inhibition of cyclooxygenase-1 and -2 in near-term and preterm fetal rats. Prostaglandins Other Lipid Mediat 2006;79:34-42.
38. Paladini D, Marasini M, Volpe P. Severe ductal constriction in the third-trimester fetus following maternal self-medication with nimesulide.Ultrasound Obstet Gynecol 2005;25:357-61.
39. Ostensen ME, Skomsvoll JF. Anti-inflammatory pharmacotherapy during pregnancy. Expert Opin Pharmacother 2004;5:571-80.
Acknowledgements
The authors thank the Pharmaceutical Assistance Service of the Valencian Health Authorities for their collaboration in documenting the data on NSAID consumption in the Valencian Community.
![]() Correspondence:
Correspondence:
Dr. Rafael Poveda Roda
Servicio de Estomatología
Hospital General Universitario de Valencia
Avda. Tres Cruces 4
46014 – Valencia (Spain)
E-mail: poveda_raf@gva.es
Received: 8-07-2006
Accepted: 5-11-2006