INTRODUCTION
Clinical trials in both adults and children have questioned the benefit of early parenteral nutrition (PN). The adult early versus late PN study from 2011 supported late initiation of PN.1 These results were incorporated into the 2016 adult critical care nutrition guidelines.2 The pediatric community was hesitant to extrapolate the adult results to children due to pediatric ontogenesis. Children have nutritional growth demands in addition to their maintenance metabolic requirements, making many reluctant to withhold parenteral nutrition to those patients not receiving goal caloric intake via the enteral route during hospitalization. Without trial results indicating otherwise, provision of earlier PN in pediatrics in comparison with adults was generally accepted.
A large pediatric multicenter, randomized controlled trial (RCT), delaying PN for one week in critically ill pediatric patients resulted in decreased length of stay, shorter duration of mechanical ventilator support, and decreased incidence of infection.3 The publication generated controversy as evidenced by letters to the editor questioning methodology and discounting the external applicability of the study along with its conclusions.4,5 Within our institution some practitioners were hesitant upon initial discussion to move toward later initiation of PN. Prior to the RCT pediatric study, practice varied within our pediatric intensive care unit (PICU) regarding when to initiate PN and was heavily dependent upon attending physician preference. Historically at our institution, many practitioners opted to initiate early PN within the first three days of admit in those patients not meeting goal caloric intake. The RCT provided outcomes based evidence supporting a later initiation of PN than was customary at our site.
Primary literature is a catalyst for evaluation of local, cultural prescribing and can lead change in clinical practice. Primary literature also influences national consensus recommendations, although guideline changes occur over a longer time period and often lag local changes already made in response to study publication. The large RCT included objective outcomes favoring delayed PN and provided impetus for practice change and greater standardization regarding timing of PN initiation at our institution.
At the time of the pediatric early versus late PN publication in 2016 outlining the benefits of late PN initiation, pediatric critical care guidelines were absent of recommendations regarding timing of PN initiation. Given the available primary literature, the PICU and nutrition support team moved to develop a more consistent approach in the timing for initiation of PN in the critically ill child. The objective of our healthcare improvement initiative was to decrease the percentage of patients initiated on PN prior to day five within the PICU.
METHODS
Inclusion
We performed a retrospective, pre/post quasi-experimental study of patients admitted to the PICU during the eighteen month period between July 1, 2016 and December 31, 2017. Our comparator group included patients admitted to the PICU between January 1, 2015 and June 30, 2016. The patients were required to be admitted to the PICU within the first 24 hours of admission and be greater than or equal to 30 days old at hospital admit.
Exclusion
Patients admitted to the PICU currently receiving PN were excluded. Patients within the NICU and cardiac intensive care unit (CICU) were excluded. NICU patients were excluded, as the quality initiative was based on a large RCT for PICU patients which excluded preterm neonates. CICU patients were excluded because our CICU was in process of conducting a quality project surrounding implementation of standard feeding protocols, which included PN recommendations.
Aim
The primary aim of the project was to decrease the PN initiation rate for patients in the first four days following PICU admission. The secondary aim was to decrease the percentage of patients receiving PN three days or less. Success was defined as decreasing the percentage of patients initiated on PN prior to day five within the PICU. Surveillance of length of stay was conducted to ensure the intervention was not adversely affecting the local patient population.
Key Drivers
The key drivers were 1) collaboration between dietitian, prescriber and pharmacist and 2) dissemination of recent evidence for PN initiation within the PICU population.
Approach
During the second quarter of 2016, formal review of published RCT results evaluating early versus late PN initiation occurred within the multi-disciplinary nutrition support team composed of physician, dietitian, nurse, and pharmacy representation. Concurrent to the nutrition support team evaluation, the PICU physician group reviewed the RCT during a journal club presented by a PICU fellow. A subsequent meeting was scheduled between the two groups to determine a unified recommendation for PN initiation within the PICU. It was determined that individualized PN would be initiated on day five from PICU admission in patients unable to receive adequate enteral nutrition. We did not change our aim to initiate enteral nutrition support within 48 hours of PICU admission and achieve 60% of goal feeding rate within seven days of admission. Enteral nutrition continued to be generally provided via NG and initiated at a trophic rate (<25% of goal volume) with periodic rate advancement to goal volume within 24-48 hours.
Education
The dietitian and pharmacist jointly write PN orders for patients within the PICU, with final approval of a PN order by a physician or nurse practitioner. Formal education of pharmacy and dietitian personnel was provided during regularly scheduled meetings, regarding the rationale and decision to initiate PN on day five from PICU admission in patients failing to reach nutritional goals. A computer based required competency for dietitians was drafted to include the PN initiation recommendation. Residents rotate through the PICU at our teaching institution staffed by university attending, fellow, and resident physicians. The PICU dietitian was responsible for education of residents regarding the quality initiative upon commencement of their PICU rotation.
Implementation
The standard approach regarding recommendation of PN initiation for PICU admits on day five began third quarter 2016. The PICU dietitian and pharmacist were present for multidisciplinary rounds to make consistent recommendations regarding PN initiation. The PICU dietitian written note within the electronic medical record was updated to reflect the decision to initiate PN later than the historical PICU practice of early PN initiation. Follow up and discussion occurred between the PICU dietitian and attending when deviations to the standard approach occurred.
Outcomes
Our primary outcome measure was percentage of PICU admissions with PN initiation within the first 4 days of admission. The secondary outcome was percentage of PICU admissions with up to 3 days of PN.
The primary balancing measures were PICU days and hospital length of stay for all PICU admissions. These outcomes were calculated for all included PICU admissions as well as for PICU admissions receiving PN. Data were examined after six months and then after an additional year. Feedback was provided to dietitians, pharmacists and clinicians.
Analysis
We followed our primary and secondary measures on statistical process control (SPC) charts created using QI Macros 2017.05 (KnowWare International Inc, Denver, CO). We plotted the outcome on the vertical axis and time on the horizontal axis. Each data point represents one month of data. Charts were annotated with interventions. Separate centerlines were calculated for the baseline time period and the intervention period.
Patient characteristics, outcomes measures and balancing measures between the baseline and intervention periods were compared using Fisher’s exact test for categorical variables and Mann Whitney U for continuous variables. All analyses were performed in R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics of the early PN group, January 1, 2015-June 30, 2016, and the post-intervention group designated as delayed PN group, July 1, 2016-December 31, 2017, are contained in Table 1. A total of 2333 patients were identified in the early PN group and a total of 2491 patients in the delayed PN group. The percentage of patients receiving PN within the first four days of hospitalization was 5.5% in the early PN group versus 3.1% in the delayed PN group (p<0.001). (Figure 1) The percentage of patients receiving three days or less of PN decreased from 2.6% of patients to 1.5% (p=0.01). (Figure 2) For the subset of patients who were initiated on PN after admission to the PICU, median PICU length of stay was 7 days versus 6 days in the early PN group versus delayed PN group (p=0.32). Hospital length of stay for patients receiving PN was 18 days in the pre-intervention group and 18 days in the delayed PN group (p=0.26). (Table 2)
Table 1. Patient Characteristics.
| Early PN (pre-intervention) (n=2333) | Delayed PN (post-intervention) (n=2491) | p-value | |
|---|---|---|---|
| Age: years, median (IQR) | 3 (0,11) | 3 (1,12) | 0.006 |
| Sex: Male, n (%) | 1287 (55%) | 1417 (57%) | 0.23 |
PN= parenteral nutrition

Figure 1. Percentage of patients with parenteral nutrition initiation prior to day five from PICU admit.PICU: pediatric intensive care unit; UCL: upper control limit
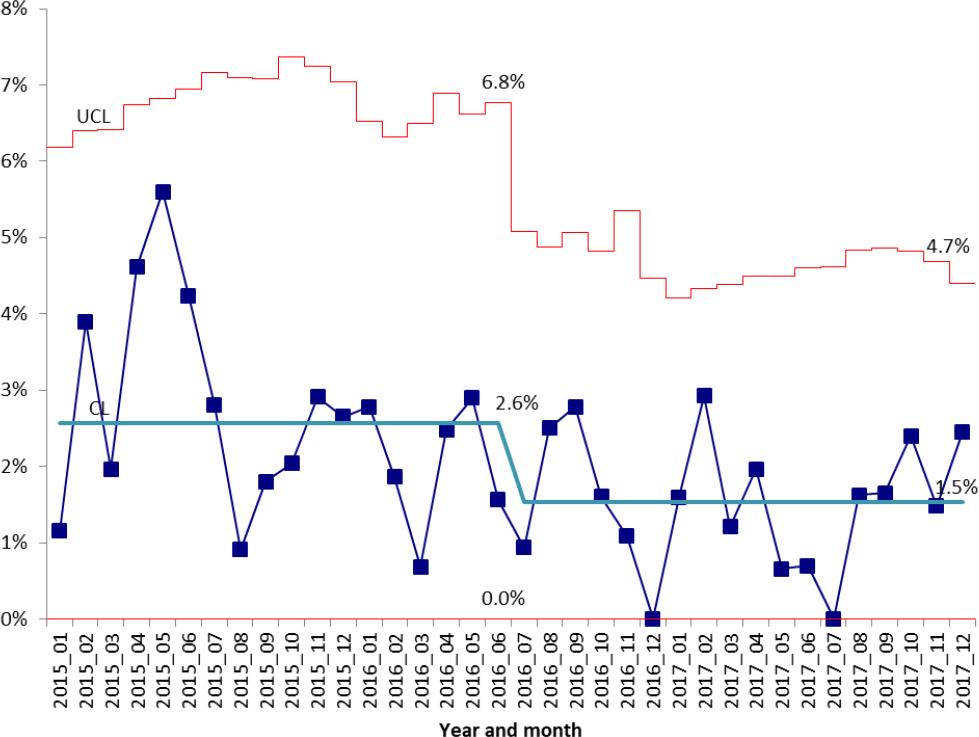
Figure 2. Percentage of PICU patients receiving parenteral nutrition for three days or less.UCL: upper control limit
Table 2. Patient Outcomes.
| Early PN (pre-intervention) (n=2333) | Delayed PN (post-intervention) (n=2491) | p-value | |
|---|---|---|---|
| PN started before day 5, n (%) | 128 (5.5%) | 77 (3.1%) | <0.001 |
| PN duration ≤ 3 days, n (%) | 60 (2.6%) | 38 (1.5%) | 0.01 |
| Balance measures | |||
| All patients | |||
| PICU LOS, days, median (IQR) | 2(2,4) | 2(2,4) | 0.06 |
| Hospital LOS, days, median (IQR) | 4 (2,8) | 4 (2,7) | 0.004 |
| Patients receiving PN | |||
| PICU LOS, days, median (IQR) | 7 (3,12.75) | 6 (2,13) | 0.32 |
| Hospital LOS, days, median (IQR) | 18 (9,28.25) | 18 (10.25,30) | 0.26 |
PN: parenteral nutrition; LOS: length of stay; PICU: Pediatric intensive care unit
DISCUSSION
The rate of PN initiation during the first four days from PICU admit decreased nearly 50% following implementation of our quality initiative. The percentage of patients receiving short term PN, defined as three days or less, likewise decreased after the implementation. Overall the pre and post intervention groups had comparable lengths of stay. Of particular interest there were similar lengths of stay when comparing only those patients who received PN during their stay, supporting the safety of the practice change. The success of this quality initiative was highly dependent upon culture change through collaboration, rather than policy making and enforcement.
In addition to measuring the compliance of the initiative, one objective of the analysis was to evaluate the safety and efficacy of the initiative. A concern expressed by some prior to starting our initiative was that those patients receiving later PN would have a negative outcome. The RCT saw a shorter length of stay for the patient population randomized to the late PN initiation group, although the majority of the late group never even received PN because they succeeded in reaching enteral goals prior the late initiation time point. Therefore the concern was that although an overall benefit to the late PN initiation group was seen in the RCT, the patients eventually needing PN might be adversely effected. In the analysis of our quality initiative we therefore focused on comparison of length of stay only in patients receiving PN, rather than the whole PICU population. Examining only those who received PN removed the hypothesized benefit that better outcomes in a delayed PN group are related to the portion of that population that were not initiated on PN. The median length of stay for those patients who received PN was similar pre and post intervention, supporting safety of the initiative relative to the length of stay outcome.
Despite the results of the RCT, debate continues over the appropriate time to initiate PN in the critically ill child, as evidenced by the recommendations released from the American Society for Parenteral and Enteral Nutrition and the Society of Critical Care Medicine the year after the primary literature publication.6 These recommendations acknowledge the study results to begin PN after seven days, but counter with expert opinion the option to begin PN 24 hours after PICU admission. Thus, current guideline recommendations lack focused specificity for standard provision of care regarding PN initiation.7 The broad national debate over appropriate PN initiation, reflects the range of opinions expressed at our facility during our initial consensus discussions. Our quality initiative began shortly after the RCT but prior to the national guideline recommendations. In an effort to provide standard care, follow published evidence and bridge competing stakeholder viewpoints, our group opted to move forward with a standard of PN initiation in patients not reaching sufficient caloric intake by day five from PICU admit. Further analysis of the original study continue to support a delayed implantation of supplemental PN.8,9,10
Applying an evidence based approach to standardize care through a quality initiative can be difficult when national consensus is varied. Keeping our primary aim in focus during weekly nutrition support meetings and supporting the key drivers of education and continued multidisciplinary collaborative discussions, led to practice change. There was statistically significant movement toward decreasing PN initiation within the first five days of PICU within our institution. Likewise, there was a decrease in the percent of patients who were initiated on PN and then only received the product for less than or equal to three days.
There is opportunity for continued improvement, as evidenced by the analysis of our quality initiative data. Although statistically significant reductions were seen in the percentage of patients initiating PN prior to PICU day five, there are still on average four percent of our patients received an earlier PN initiation than our quality initiative standard. When following up with teams on why they initiated early, a common response was that the team anticipated the patient would need PN support for an extended period of time, therefore the team did not see benefit in waiting. Yet, of those patients initiated on PN prior to PICU day five, a portion received PN three days or less. The follow up analysis of the quality initiative provides relevant information for continued discussions surrounding the provision of quality standard care as related to PN initiation. Further progress toward achieving the quality measure of PN initiation on day five could be made through formation of formal policy.
A barrier to quality initiatives to improve evidence based care is overcoming existing culture. Generally, improvement efforts concisely identify an aim, key drivers, and interventions that focus on influencing the existing practice culture to realign within a new paradigm. Examination of the quality initiative reported herein is instructive, as it was based primarily on review of primary literature, open discussion, consistent recommendation and ongoing education rather than strict policy and subsequent enforcement. Whether it be through policy and enforcement or education and recommendation the goal of any initiative is to provide consistent high quality care that is sustainable. Sustainability is often achieved through cultural change. Once culture has changed, practice change has a greater probability of continuing beyond the intensive implementation phase. Limitations of the study include the retrospective analysis at a single center and lack of analysis within specific disease state patient populations.
CONCLUSIONS
Standardizing the recommendation and approach to PN initiation after evaluation of a large published RCT decreased early and overall PN use within our PICU. Discussion, consensus gathering and consistent recommendations proved effective in the quality initiative. Evaluation of this project demonstrate that influencing change through light touch techniques can be effective, especially in cases where firm national professional organization guidance is not available due to varying stakeholder opinions. Safety, as related to length of stay evaluation, was also demonstrated through the quality initiative evaluation.














