Introduction
The use of colonoscopy for routine screening for colon and rectal cancer has been one of the most successful public health projects worldwide. Although colonoscopy is the most frequent technique that requires a proper bowel preparation, it could be necessary in others diagnosis tests: CT colonography, barium enema and surgery on the gastrointestinal tract. Effective preparation requires an adequate level of cleansing.
The osmotically balanced, polyethylene glycol-based electrolyte solutions are elected because they are safer and faster than large volume saline-based electrolyte solutions. Many patients have great difficulty to drink the large volume, that can result in unsuccessful preparation and poorly cleansed colon, and therefore, inadequate study assessment.1 The small volume of oral sodium phosphate solution (OSP) was associated with improved patients compliance, less discomfort, and superior colonic cleansing.
The OSPS is not exempt from risks. It can cause transient hyperphosphatemia and can induce intravascular volume contraction. There have been reports of serious adverse events, including death, and there have been case reports of nephrocalcinosis with renal failure associated with the use of oral sodium phosphate.2-4 In fact, in 2008 FDA issued a warning about the use of OSP in patients with chronic kidney disease (CKD) or major cardiovascular comorbidities.
Unfortunately, the results of the available epidemiologic data from large populations are quite confusing some studies reporting a potent association5-7 and others reporting non-significant trends toward better kidney outcomes after OSP compared with other purgatives.2,8 A major limitation of most of theses studies is they just consider renal damage when an acute renal failure happens. However the chronic damage has been well documented with the use of this purgative solution.9,10
Given the large number of patients exposed to OSP annually, clarification of this association is compelling. We present a series of patients with acute and chronic renal damage link to use of OSP.
Material and methods
Patient selection and study design
From 2006 to 2009 we identified 12 patients with suspected nephropathy after colonoscopy prepared by phosphosoda. The clinical suspicion of PN (was based on the presence of an acute o chronic renal function deterioration (>50% from basal serum creatinine) chronologically related to colonoscopy. In all the patients, the presence of proteinuria o hematuria was ruled out by repeated urine analysis, also presence of urinary tract obstruction or other urinary tract abnormalities was excluded by appropriate radiological examinations. Patients with clinical or analytical data that suggested systemic diseases were excluded.
All included patients had at least one Scr determination in the three months before the colonoscopy and it was considered as baseline Scr. The highest Scr value registered was recorded, as well as the need for acute dialysis. The creatinine concentration at 6 months after the procedure was recorded.
Medical records of the patients were reviewed for this study. We reviewed for age, gender, medical history (high blood pressure, smoker, hypercholesterolemia and diabetes mellitus) and medication (ACE inhibitors, diuretics). Estimated glomerular filtration rate (eGFR) was calculated according to the MDRD4 formula. All cases happened in a single hospital. The Scr was measured in a single laboratory and by the kinetic Jaffé blank correction method.
Final Scr was defined as the value obtained 6 months after withdrawal of the offending drug. An incomplete recovery of baseline renal function was defined by an Scr value higher than at least 25% of the baseline value.
The recommended regimen of OSP solution (Casen Fleet Laboratories) consisted of two 45 ml doses taken 10-12 h apart, the evening before and the morning of colonoscopy. Each 45 ml dose contained 21.6 g of monobasic sodium phosphate (NaH2PO4) and 8.1 g of dibasic sodium phosphate (Na2H-PO4), which is equivalent to 5.8 g of elemental phosphorus. The 5.8 g of phosphorus was diluted into a single eight ounce glass and administered twice in a 12-24 h period (total dose 11.6 g of phosphorus).
Statistical analysis
The baseline characteristics are expressed as means ± standard deviation (SD). The groups were compared using the 2-sample t test, χ2-test and Fischer exact tests, respectively. The 2-sample t test was used to compare the creatinine level and eGFR between the study and control groups. The χ2-test was used to compare group characteristics of diabetes, hypertension, and use of angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors and diuretics. P < 0.05 was considered significant. Statistics were calculated using SPSS for Windows, version 11 (SPSS Inc., Chicago, IL, USA).
Results
Twelve cases of phosphate nephropathy were diagnosed: four of them were confirmed by renal biopsy. The baseline characteristics of patients in both groups are shown in Table 1.
Table 1 Baseline clinical characteristics of 12 patients with phosphate nephropathy.
| Age (years) | Gender | eGFR (ml/min/1.73m2 )© | Hypertension | Diabetes mellitus | ACEi-ARB | Diuretics | Renal biopsy | Course | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 61 | Man | 63.6 | No | No | No | No | Yes | Acute |
| Case 2 | 85 | Female | 41.3 | Yes | Yes | No | Yes | - | Chronic |
| Case 3 | 71 | Man | 70.7 | Yes | Yes | Yes | Yes | Yes | Acute |
| Case 4 | 73 | Female | 73.2 | Yes | Yes | Yes | Yes | - | Chronic |
| Case 5 | 84 | Man | 59.6 | Yes | No | Yes | Yes | - | Chronic |
| Case 6 | 59 | Female | 137.9 | Yes | No | Yes | Yes | - | Chronic |
| Case 7 | 70 | Female | 95.6 | Yes | Yes | Yes | Yes | Yes | Chronic |
| Case 8 | 72 | Man | 54.5 | Yes | No | Yes | Yes | - | Chronic |
| Case 9 | 72 | Man | 54.5 | Yes | No | Yes | Yes | - | Acute |
| Case 10 | 73 | Female | 65.6 | Yes | No | Yes | No | Yes | Chronic |
| Case 11 | 75 | Man | 54.0 | Yes | No | Yes | No | - | Chronic |
| Case 12 | 92 | Man | 35.2 | Yes | No | Yes | No | - | Chronic |
© Modification of the diet in renal disease (MDRD)-4 equation was used to estimate glomerular filtration rate (eGFR).
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
There were a predominance of cases of men, 68.2% with mean age 69.44 ± 7.2 years. The main co-morbidities in our patients were high blood pressure 91.6% and DM 33.3%. The 73.3% of the patients received treatment with ACE inhibitors or ARBs, and the 66.5% received treatment with diuretics.
All patients received just one dose of OSP. None of them were subjected to repeated cleanings intestinal in a short period. One patient undergone endoscopy procedures during hospitalization for acute indications (case 1). The other eleven patients received the OSPS preparation and performed the colonoscopy in outpatient regimen.
Kidney function
Before the procedure, eGFR was >60 ml/min/1.73 m2 in 7 patients. CKD stage 3a was present in 3 patients and CKD 3b in 2 patients. None has CKD 4 or 5 stage.
Three patients debuted as acute renal failure (two cases confirmed with renal biopsy) and nine patients developed chronic renal damage (two cases confirmed with renal biopsy). No significant differences were found in the baseline renal function, age, sex, treatment with ACEi or ARBs, diuretics, mellitus diabetes or hypertension.
In the group of patients who debuted as AKI (acute kidney injury), one of patients begun with nausea and vomiting hours after colonoscopy (case 1). The others two patients debuted in the week after the OSP administration. In the patients with delayed debut the average time until the diagnosis was 54 days (15-120 days). All of them were asymptomatic or pauci symptomatic and the renal damage was an accidental diagnosis.
Patients presented with AKI had a higher initial loss of glomerular filtration rate, but no significantly, than the patients with chronic presentation (52 ± 9.9 ml/min/1.73 m2 vs 38 ± 24 ml/min/1.72 m2, NS).
Calcium and phosphorus levels were normal in the delayed analysis. None other alterations were finding.
Renal follow up
One patient of the acute course group needed substitutive renal therapy in the moment of diagnosis, without later recovery. Six months later, none of the remainder two patients of this group recovered completely the glomerular filtration rate, although they got it partially. The final average loss was 22.5 ± 9.7 ml/min/1.73 m2.
In the group with delayed presentation, six months later of colonoscopy just two patients recovered renal function as previous baseline and nine patient did not improved. In the patients who did not recovered baseline renal function, the final average loss of glomerular filtration rate was 26 ± 20 ml/min/1.73 m2 (Table 2).
Table 2 Renal outcome in 12 patients with phosphate nephropathy.
| Course | Baseline eGFR (ml/min/1.73m2 ) | Peak eGFR (ml/min/1.73m2 ) | eGFR 6 month after (ml/min/1.73m2 ) | ΔeGFR 6m after (ml/min/1.73m2 ) | |
|---|---|---|---|---|---|
| Case 1 | Acute | 63.6 | 7.9 | 41.6 | 22.0 |
| Case 2 | Chronic | 41.3 | 30.2 | 48.2 | −6.9 |
| Case 3 | Acute | 70.7 | 9.9 | HD | HD |
| Case 4 | Chronic | 73.2 | 36.9 | 50.5 | 22.7 |
| Case 5 | Chronic | 59.6 | 28.6 | 38.3 | 21.3 |
| Case 6 | Chronic | 137.9 | 51.9 | 76.5 | 61.4 |
| Case 7 | Chronic | 95.6 | 29.7 | 39.2 | 56.3 |
| Case 8 | Chronic | 54.5 | 15.3 | 19.6 | 34.9 |
| Case 9 | Acute | 54.5 | 12.8 | 31.5 | 23.0 |
| Case 10 | Chronic | 65.6 | 8.6 | 27.3 | 38.3 |
| Case 11 | Chronic | 54.0 | 20.7 | 31.0 | 23.1 |
| Case 12 | Chronic | 35.2 | 18.0 | 23.3 | 11.9 |
HD: hemodialysis.
Renal biopsy findings
In four patients a biopsy was performed. The renal biopsy findings in PN primary involve the tubules and were dependent on the time interval between the preparation with oral sodium phosphate and renal biopsy. The biopsy from patients with AKI acute degenerative changes predominated a resemble findings seen an acute tubular necrosis.11 The renal biopsies performed in patients with chronic course showed tubular atrophy and interstitial fibrosis. Regardless of the degree of acuity of chronicity, the hallmark of PN was abundant tubular and less prominent interstitial calcium phosphate deposits (Fig. 1). The calcifications do not polarize and have a strong histochemical reaction with the von Kossa stain, indicating that they are composed of calcium phosphate.
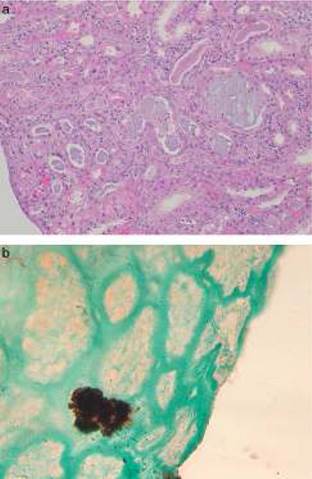
Fig. 1 Renal biopsy findings in chronic phosphate nephropathy. (a) Case of chronic phosphate nephropathy with abundant intraluminal and intracellular calcifications in distal tubules accompanied by tubular atrophy and fibrosis (hematoxylin and eosin) and (b) a positive histochemical reaction with the von Kossa stain confirms that the tubular concretions are composed of calcium phosphate.
Discussion
Ten years ago some cases of PN began to be published.4 The former cases were presented as an acute illness that manifested as tetany, acute kidney disease or cardiovascular collapse usually within hours of bowel preparation. Patients had marked hyperphosphatemia and hypocalcemia and some patients required even hemodialysis.12
Although case reports and case series provide strong support for an etiological relationship between OSP and the development of PN, epidemiological studies have showed less consistent results. In an retrospective observational study of 9799 patients who filled a prescription for an OSP or PEG (polyethylene glycol), 114 patients (1.16%) developed AKI: 1.29% of the 6432 patients who received OSPS and 0.92% of the 3367 patients who received PEG. The PEG group included patients who were significantly older and had a higher incidence of diabetes mellitus, hypertension, CHF, CKD, diuretics use and ACEi or ARBs use (p < 0.05). In the multiple logistic regression models adjusted for covariates and suspected risk factors, OSP was found to be the strongest risk factor for the development or AKI after colonoscopy (OR 2.35, CI 95% 1.51-3.66) when compared with PEG. When a more “strict” definition of AKI was considered (doubling of serum creatinine vs >50% serum creatinine increase), the OSP purgative use remained significantly associated with increased risk for AKI (OR 3.52, CI 95% 1.13-10.93).5
In a recent, large retrospective cohort study using a US-based administrative claims database. The number of the patients included was impressive: 121,226 received OSP pill preparation and 429,430 received PEG. Patients were followed up to 6 months for AKI, renal failure and dialysis. The investigators did not find any increase risk of AKI in OSP pill users compared with PEG (0.2% vs 0.3%). The investigators acknowledged that the study of administrative claims for AKI outcomes may be an insensitive measure of renal dysfunction.8
Brunelli et al. undertook a systematic review and metaanalysis that concluded that it is not possible to discern if an association between OSP exposure and AKI exits. The use of different AKI definitions and significant heterogeneity among the studies referred to choice of bowel preparation, patients populations, baseline renal function, limited the result of the study.13
The chronic form of kidney damage due to OSP agents may present weeks after the initial exposure and may be identified as the result of an investigation of mild or non-specific symptoms such as fatigue. Sometimes, it is just discovered by a casual analysis and the anamnesis is the clue. This was the most frequent pattern in our study. The 65% of the patients were referred for study by nephrologist for increasing in serum creatinine in casual analysis.
This form of presentation requires the inclusion of this entity in the differential diagnosis of CKD. The nephrologist or physician who treats these patients should consider the possibility of PN in patients with renal damage without proteinuria or hematuria when the patient has undergone a digestive test prepared OSD.
Whereas until now to estimate the incidence of kidney disease for OSP we consider just the case presented as AKI, ignoring cases of delaying presentation. It may mean that this problem is more prevalent than is currently recognized. In fact, in the studies that analyze the safe of bowel preparation for colonoscopy the follow up was too short to identify it.
But, the true issue of this complication is not to know the AKI incidence if the secondary chronic loss of renal function over time. The outcomes of these patients are very inconstant: in most of cases of our study represent a substantial and irreversible loss of glomerular filtration. In Hurst et al.,5 just the 16% of patients returned to baseline renal function. They could not know if any of these patients requires renal replacement. In cases reported at least four patients developed ESRD.14 In most of studies this information is not available. In our study one patient (case 3) required renal replacement at diagnosis time and she did not recover renal function. The other patients had an average chronic loss glomerular filtration > 20 ml/min/1.72 m2.
Different options to get an adequate level of cleansing, such as PEG, can also cause renal damage.5,15 The AKI incidence reported for PEG vary between 0.92% and 6%. If we consider that the damage is mainly due to renal hypoperfusion by dehydration, we could think that this injury would less severe and more reversible, although to our knowledge, none study has analyzed the long lasting effect of this injury.
The presence of CKD before the colonoscopy has been considered as the most weighty risk factor to develop kidney damage.6,14,16 However in our study the 63% of the patients had an eGFR > 60 ml/min/1.73 m2. In fact, a lot of cases with normal o nearly normal baseline renal function with kidney injury induced OSP have been published.17 Some studies have suggested that females, above all elderly females, are at greater risk than males for developing PN.14 They might have reduced renal capacity of handle a phosphate load and their smaller body mass makes then more sensitive to fluid loss. This loss of volumen in conjunction with limited oral intake dictated by precolonoscopy protocols, may exacerbate some of the electrolyte abnormalities and the risk of renal failure among patients receiving these agents. Drugs that impact intravascular volumen and renal perfusion may increase the risk of kidney injury after OSP use, including diuretics, ACE inhibitors, angiotensin receptor blockers and non-steroidal anti-inflammatory agents.
The standard dose of OSP contains 11.6 g of phosphorus. About 57% of the dose is excreted in feces, 15% in urine and 28% is retained in the body for 24 h.18 The total amount of phosphate increases after the first and second doses to fourfold and eightfold of the baseline level respectably and remains elevates at 24 h. These results suggest that the second dose of OSP is particularly dangerous because renal reabsorption of phosphate is fully inhibited at the time of dosing and thus the urinary concentrations of phosphate remains elevated for prolonged period.
In summary, the administration of oral sodium phosphate can cause an intense renal injury, sometimes depend of dialysis and often irreversible. This side effect does not always occur in high-risk patients and the most common pattern of presentation was renal chronic disease. The series that study this adverse effect should consider both the acute and chronic pattern damage.














