Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Nutrición Hospitalaria
versão On-line ISSN 1699-5198versão impressa ISSN 0212-1611
Nutr. Hosp. vol.28 no.5 Madrid Set./Out. 2013
https://dx.doi.org/10.3305/nh.2013.28.5.6679
ORIGINAL / Otros
Validity and reliability of the Dietary Sodium Restriction Questionnaire (DSRQ)
Validez y fiabilidad del Dietary Sodium Restriction Questionnaire (DSRQ)
Karina S. M. d'Almeida1,2, Gabriela C. Souza2,3 and Eneida Rejane Rabelo-Silva1,2,4
1Federal University of Rio Grande do Sul. Graduate Program in Health Sciences. Cardiology and Cardiovascular Sciences
2Hospital de Clínicas de Porto Alegre. Cardiology Division. Heart Failure Clinic
3Federal University of Rio Grande do Sul. School of Medicine. Department of Internal Medicine
4Federal University of Rio Grande do Sul. School of Nursing. Porto Alegre. RS. Brazil
Funding Sources: Coordenacação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE-HCPA)
ABSTRACT
Introduction: The Dietary Sodium Restriction Questionnaire (DSRQ) was designed to assess attitudes and behaviors of patients with heart failure (HF) related to following a low-sodium diet. Recently, it has been translated and culturally adapted for use in Brazil. However, further validation of the instrument is required before it can be used in the management of patients with HF in Brazil.
Objective: To test the reliability and validity of the Brazilian version of the DSRQ.
Methods: Face and content validity were assessed by a panel of experts. Construct validity was tested using exploratory and confirmatory factor analysis. Reliability was tested using Cronbach's alpha to assess the internal consistency of the instrument.
Results: A total of 206 systolic HF outpatients were assessed (mean age, 60.4 ± 11.9 years). Face and content validity analysis showed equivalence between the Brazilian version and the original instrument. In the exploratory factor analysis, the principal component analysis (PCA) yielded four factors with eigenvalues greater than 1. Three models were tested in the confirmatory factor analysis, and the three-factor model resulting from the PCA showed the best fit, accounting for 49% of the variance. Alpha values obtained for the attitude/ subjective norm, perceived behavioral control, and dependent behavior subscales were 0.71, 0.67, and 0.79, respectively.
Conclusions: Our results suggest that the final validated Brazilian version of the DSRQ is a valid and reliable tool for measuring attitudes and behaviors related to following a low-sodium diet in Brazilian patients with HF.
Key words: Validation studies. Questionnaires. Dietary Sodium. Heart failure. Dietary sodium restriction questionnaire.
RESUMEN
Introducción: El Dietary Sodium Restriction Questionnaire (DSRQ) evalúa actitudes y comportamientos de pacientes con insuficiencia cardiaca (IC) relacionados con el cumplimiento de la restricción de sodio. Recientemente, ha sido traducido y adaptado culturalmente para uso en Brasil. No obstante, una validación adicional del instrumento se requiere para que pueda ser utilizado en el manejo de pacientes con IC en Brasil.
Objetivo: Probar la fiabilidad y validez de la versión brasileña del DSRQ.
Métodos: Validez aparente y de contenido fueron evaluados por un grupo de especialistas. Validez de cons-tructo se evaluó mediante análisis factorial exploratoria y confirmatoria. La fiabilidad y consistencia interna del cuestionario fue evaluada mediante el coeficiente alfa de Cronbach.
Resultados: Un total de 206 pacientes ambulatorios con IC fueron evaluados (edad media, 60,4 ± 11,9 años). Los resultados de la validez aparente y de contenido demostró la equivalencia entre la versión brasileña y de la versión original. En el análisis factorial exploratorio, el análisis de componentes principales (PCA) se obtuvieron cuatro factores con valores superiores a 1. Tres modelos fueron probados en el análisis factorial confirmatoria, y el modelo de tres factores resultantes del PCA mostró el mejor ajuste, representando 49% de la varianza. El alfa obtenido para las escalas de actitud/norma subjetiva, control de la conducta percibido y comportamiento dependiente fueron 0,71, 0,67 y 0,79, respectivamente.
Conclusiones: Nuestros resultados sugieren que la versión brasileña del DSRQ es un instrumento válido y fiable para medir las actitudes y comportamientos relacionados con una dieta baja en sodio en pacientes brasileños con IC.
Palabras clave: Estudios de validación. Cuestionarios. Sodio en la dieta. Insuficiencia cardíaca. Dietary sodium restriction questionnaire.
Abbreviations
HF: Heart failure.
DSRQ: Dietary Sodium Restriction Questionnaire.
KMO: Kaiser-Meyer-Olkin test.
PCA: principal component analysis.
Introduction
The prescription of a low-sodium diet is a fundamental component of nonpharmacologic therapy in patients with heart failure (HF).1-4 However, dietary sodium nonadherence is extremely common, and excessive sodium intake remains a leading cause of decompensation and hospital admissions in this population.5-7
The Dietary Sodium Restriction Questionnaire (DSRQ) was designed to specifically measure attitudes and behaviors of patients with HF toward adherence to a low-sodium diet.8 The instrument is based on the theory of planned behavior and assesses adherence through the use of three subscales (attitude, subjective norm, and perceived behavioral control), allowing health professionals to better understand the reasons behind nonadherence to this recommendation.8
Recently, the DSRQ has been translated into Portuguese and culturally adapted for use in Brazil.9 However, further validation of the instrument is still required before it can be widely used in the management of patients with HF in this new setting. A key feature of validation studies of cross-culturally adapted instruments is to confirm whether the statements contained in the translated version can successfully reproduce the semantic content of the original text in order to preserve the original meaning and achieve the same effect in the target text.10-12
Objective
This study aimed to test the psychometric properties of the Brazilian version of the DSRQ for the measurement of attitudes and behaviors of Brazilian patients with HF toward adherence to a low-sodium diet.
Methods
Study design and population
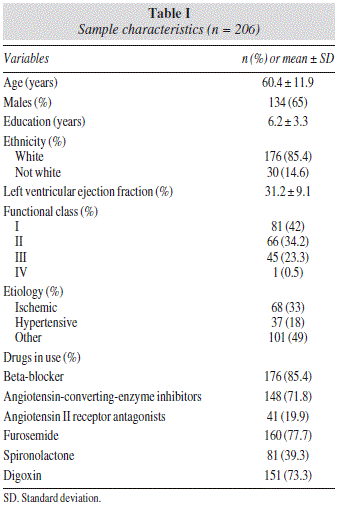
This methodological study was conducted at a university hospital located in southern Brazil. Men and women attending the HF outpatient clinic of our institution between March 2010 and June 2011 were eligible for participation in the study if they were aged ≥ 18 years and had a diagnosis of HF and left-ventricular systolic dysfunction (defined as ejection fraction ≤ 45%). The study was approved by the Research Ethics Committee of the institution (Institutional Review Board-equivalent) and was conducted in accordance with the provisions of the Declaration of Helsinki. All participants provided written informed consent prior to their inclusion in the study.
Data collection
The study subjects were invited to participate during outpatient visits. Data were collected via individual interviews conducted as part of the patient's medical assessment. Individual interviews lasted approximately 10 minutes. Sociodemographic and clinical characteristics of the sample were also recorded.
The Dietary Sodium Restriction Questionnaire (DSRQ)
The DSRQ is an assessment instrument used to measure patients' perceptions of their barriers to, and attitudes toward, following a low-sodium diet.8 The original instrument is divided into three subscales: 1) attitude; 2) subjective norm; and 3) perceived behavioral control. The attitude subscale comprises six items and assesses the patient's beliefs on the results of adopting a given behavior. The subjective norm subscale comprises three items and refers to the importance of the patient's perception that others approve or disapprove of performing the behavior. Finally, the perceived behavioral control subscale comprises seven items and evaluates the patient's ability to identify facilitators and barriers related to the behavior.8
The DSRQ has already been translated into Portuguese and culturally adapted for use in Brazil. The development, translation and cross-cultural adaptation process of that version of the DSRQ has been previously described.9 Briefly, items and subscales are arranged similarly to those of the original version. Responses are recorded on a five-point Likert scale, with endpoints of "strongly disagree" (1) to "strongly agree" (5) for the first (attitude) and second (subjective norm) subscales, and "not at all" (1) to "a lot" (5) for the third (perceived behavioral control) subscale. Within each subscale, individual item scores are summed up to give a total score, ranging from 6 to 30 for the attitude subscale, from 3 to 15 for the subjective norm subscale, and from 7 to 35 for the perceived behavioral control subscale.
The pretest version was applied to 44 outpatients with HF to assess the internal consistency of the instrument, obtaining good internal consistency (Cronbach's alpha = 0.77); and then applied to another sample of 40 patients with HF for interobserver agreement, with kappa values > 0.6 (0.62-1.00), demonstrating the reliability and reproducibility of the instrument.9
In addition, the questionnaire includes 11 initial items that are not part of any of the subscales submitted to validation in the present study. Those 11 items are used for descriptive purposes only and provide information on the prescription (or not) of a low-sodium diet, on the patient's difficulty following these recommendations, and on the degree to which the patient believes that the diet has helped control the disease.8
Assessment of psychometric properties
The methodological procedures of instrument validation were carried out as recommended in the literature.12
Face and content validity were assessed by a panel of experts consisting of three nutritionists, a nurse, and a specialist in linguistics. Face validity is concerned with the extent to which the instrument appears to measure the construct it was actually designed to measure. Based on previous studies 13, relevant questions used to assess face validity included: "What do patients think is measured by the scale?" and "Do patients understand the statements presented?". Content validity, in turn, examines the relevance of statements for the adequate representation of the contents addressed by the instrument.11 In this study, face and content validity were determined by further analyzing the questions and patients' responses, and revisions were made to the first version of the questionnaire if necessary.
Construct validity is concerned with the relationship between the test and the theoretical construct of interest. In this study, construct validity was tested using exploratory and confirmatory factor analysis and principal component analysis (PCA) to assess the unidimensionality of the construct under investigation and to examine statements with multiple underlying dimensions (subscales).12
Reliability analysis focuses on the degree of consistency with which the instrument measures the attribute. At this stage, it is possible to investigate whether the items of the instrument are positively related with one another. In this study, reliability was tested using Cronbach's alpha to assess the internal consistency of the instrument 14,15.
Data analysis
Continuous variables were expressed as means ± standard deviation. Exploratory analysis with PCA and confirmatory factor analysis were performed. PCA applicability was assessed using Bartlett and Kaiser Meyer-Olkin (KMO) tests. Varimax rotation was used to allow a better interpretation of the exploratory analysis.
PCA and reliability statistical analyses were performed using the Statistical Package for the Social Sciences version 18.0. Confirmatory factor analysis was performed using the Mplus software.16 The level of significance was set at P ≤ 0.05.
Results
The sample comprised 206 patients. Mean age was 60.4 ± 11.9 years, and most patients were male (65%). Mean ejection fraction was 31.2 ± 9.1%, and 33% of the patients had HF of ischemic etiology. Clinical characteristics of the sample are described in table I.
Face and content validity analysis showed equivalence between the Brazilian version and the original instrument. During this stage, item no. 21 was further reformulated by adding extra information to the first version of the questionnaire9 with the aim of improving patient understanding, as follows (text in italics): "21. Don't understand (the importance of controlling salt) or know how (I eat out at a restaurant or another person cooks and I can't control the amount of salt)".
Regarding the scores obtained in the three subscales of the Brazilian version of the DSRQ, the first two subscales yielded values close to the upper limit (attitude: 29.0 ± 2.5; subjective norm: 13.6 ± 2.4), whereas the perceived behavioral control subscale presented lower scores (13.7 ± 6.4).
Construct validity
Exploratory PCA and confirmatory factor analysis were performed. The KMO test resulted 0.71, and the Bartlett test yielded statistically significant results (P < 0.001). The PCA yielded four factors with eigenvalues greater than 1, which accounted for 25, 14.7, 9.6, and 8.7% of the total variance, respectively. The scree plot analysis revealed a slight drop in eigenvalues after the third factor, suggesting the presence of four factors.
Three models were tested in the confirmatory factor analysis: two models originating from the PCA (three-and four-factor models) and another three-factor model in which the items of each component replicated the original questionnaire. After analysis, the three-factor model of the PCA was considered the best-fit model (table II).
Varimax rotation revealed a simple structure and items with high values for one of the components. Only three items, no. 16, 17, and 26, presented high factor loads simultaneously for two components (table III). The combined assessment of the three components accounted for 49.4% of the variance: component no. 1 accounted for 25%, component no. 2 for 14.7%, and component no. 3 for 9.6%. Thus, component no. 1 included items 12 to 20; component no. 2, items 21, 22, 26, and 27; and component no. 3, items 23, 24, and 25. Items 16 and 17 were included in component no. 1, and item 26, in component no. 2, as defined by the authors.
Reliability
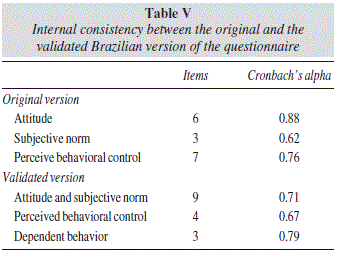
At this stage, the internal consistency of the adapted three-component Brazilian version of the DSRQ obtained in the confirmatory factor analysis was assessed. Component no. 1 (attitude and subjective norm subscale) comprised items 12 to 20; component no. 2 (perceived behavioral control), items 21, 22, 26, and 27; and component no. 3 (dependent behavior), questions 23, 24, and 25. A copy of this new version of the Brazilian questionnaire is available as a supplementary material (Appendix A). Cronbach's alpha values were calculated for each questionnaire item (table IV). Alpha values obtained for the attitude and subjective norm, perceived behavioral control, and dependent behavior subscales were 0.71, 0.67, and 0.79, respectively (table V).
Discussion
The instrument validated in the present study is the first questionnaire designed to assess attitudes and behaviors of patients with HF related to following a low-sodium diet in Brazil. This is also the first validation of the DSRQ in another language.
According to the confirmatory factor analysis, the three-component model of the PCA showed the best fit. Items were highly correlated, but their distribution resulted differently from the original instrument.8 For example, items 18, 19, and 20 belong to the subjective norm subscale in the original instrument, but were merged into the attitude/subjective norm subscale in the validated Brazilian version of the DSRQ. However, these changes did not alter the purpose of the subscales, once both assess factors that will influence the behavior of following or not a sodium-restricted diet8,17.
Conversely, items 23, 24, and 25, originally belonging to the perceived behavioral control subscale, formed a new subscale (dependent behavior). The three items in the new subscale are related to decision-making situations, e.g. at the grocery and at restaurants. Previous studies have reported that most patients are unaware of the sodium content of processed foods and that dietary restrictions may interfere with the patient's social life.18-20 These limitations have been identified as barriers to patients' adherence to low-sodium diets.21,22
The high scores observed for the attitude/subjective norm subscale of the validated version indicate that patients are able to identify signs and symptoms suggestive of excessive sodium intake, and that adherence to the restrictive diet is strongly influenced by the opinion of other people. The values found for the perceived behavioral control subscale suggest that patients face difficulties following a sodium- restricted diet, which justifies the decision to rearrange some items and compose a new subscale. Patients often adhere to dietary sodium restriction in general, but have their adherence affected when faced with decision-making situations outside their homes. It was not possible to compare the scores obtained in our patients with data from the literature because there are no similar data available about the original scale,8 and no other validation studies of the DSRQ have been found.
Changes in the arrangement of questions in the subscales may have been caused by demographic and cultural differences between the populations assessed with the original and the Brazilian version of the instrument. Among such differences, education level deserves special mention: in our sample, individuals had a mean of 6.2 years of schooling, vs. 11.8 years in the original study sample.8 This difference may have influenced patients' understanding while answering the questionnaire. Moreover, the original sample included more severe patients (48% in functional class III vs. 22% in our sample) and a higher percentage of women (44 vs. 35%). These characteristics may also have influenced patients' responses, as previous studies have indicated that patients with more severe HF have more knowledge about nonpharmacologic measures and that female patients tend to adhere more closely to dietary sodium restriction recommendations23,24.
Based on our results, we have proposed a rearrangement of items in the validated version of the instrument (table V). As a result, the final validated Brazilian version of the DSRQ (Appendix A) comprises three subscales: a) attitude and subjective norm; b) perceived behavioral control; and c) dependent behavior. The first subscale, composed of nine items, assesses patients' beliefs regarding the results obtained with adopting the behaviors listed, as well as the importance of the patient's perception that others approve or disapprove of performing the behavior. The second subscale, comprising four items, assesses the patient's ability to identify facilitators and barriers related to the behavior. Finally, the third subscale, including three items, assesses situations that require patients' decision-making outside their home.
This final version was submitted to reliability analysis. Comparison of the alpha values obtained in the three subscales of the Brazilian version (0.71, 0.67, and 0.79) with those of the original questionnaire (0.88, 0.62, and 0.76)8 indicate that the instrument remained consistent, in spite of the different arrangement of items. Although the alpha value of the perceived behavioral control subscale can be considered relatively low (0.67),25 item-total correlation was greater than 0.3 for all questions included (0.35 to 0.46), suggesting that they are correlated with one another and that they measure the same attribute.26 It is important to emphasize that alpha values are directly influenced by the number of items included in a scale, which may also explain the low values obtained.12
The factor analysis conducted in this study showed that the adapted three-component version was adequate to the reality of the population under investigation. The three newly formed subscales were considered to successfully account for the different situations that may affect patient adherence to a low-sodium diet.
Nonadherence to dietary sodium restriction remains a leading cause of decompensated HF. Therefore, a better understanding of the factors regulating adherence to dietary sodium restriction should be among the main goals of research teams, so that individual interventions can be adequately planned and implemented. Instruments such as the present version of the DSRQ can improve the investigation of such aspects.
Conclusions
The results of this study suggest that the final validated Brazilian version of the DSRQ is a valid and reliable tool for measuring attitudes and behaviors related to adherence to dietary sodium restriction in Brazilian patients with HF. Validation studies as the present one are important because they provide the international audience with valid instruments that can be used to guide interventions in clinical practice. Further validation studies may be desirable to explore cultural dietary patterns and food choices across different Brazilian regions.
Acknowledgments
The authors would like to acknowledge the researchers Brooke Bentley and Terry A. Lennie, as well as the other authors, for granting authorization for the translation and adaptation of the DSRQ into Brazilian Portuguese.
None of the authors had a conflict of interest.
References
1. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis, GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 53 (15): e1-e90. [ Links ]
2. Bocchi EA, Marcondes-Braga FG, Bacal F, Ferraz AS, Albuquerque D, Rodrigues Dde A, Mesquita ET, Vilas-Boas F, Cruz F, Ramires F, Villacorta H Jr, de Souza Neto JD, Rossi Neto JM, Moura LZ, Beck-da-Silva L, Moreira LF, Rohde LE, Montera MW, Simoes MV, Moreira Mda C, Clausell N, Bestetti R, Mourilhe-Rocha R, Mangini S, Rassi S, Ayub-Ferreira SM, Martins SM, Bordignon S, Issa VS. Sociedade Brasileira de Cardiologia. Atualização da Diretriz Brasileira de Insuficiência Cardiaca Crônica - 2012. Arq Bras Cardiol 2012: 98 (1 Suppl. 1): 1-33. [ Links ]
3. Beich KR, Yancy C. The heart failure and sodium restriction controversy: challenging conventional practice. Nutr Clin Pract 2008; 23 (5): 477-86. [ Links ]
4. Lennie TA, Worrall-Carter L, Hammash M, Odom-Forren J, Roser LP, Smith CS, Trupp R, Chung ML, Moser DK. Relationship of Heart Failure Patients' Knowledge, Perceived Barriers, and Attitudes Regarding Low-Sodium Diet Recommendations to Adherence. Prog Cardiovasc Nurs 2008; 23 (1): 6-11. [ Links ]
5. Ambardekar AV, Fonarow GC, Hernandez AF, Pan W, Yancy CW, Krantz MJ. Characteristics and in-hospital outcomes for non adherent patients with heart failure: findings from Get With The Guidelines-Heart Failure (GWTG-HF). Am Heart J 2009; 158 (4): 644-52. [ Links ]
6. van der Wal MH, van Veldhuisen DJ, Veeger NJ, Rutten FH, Jaarsma T. Compliance with non-pharmacological recommendations and outcome in heart failure patients. Eur Heart J 2010; 31 (12): 1486-93. [ Links ]
7. Arcand J, Ivanov J, Sasson A, Floras V, Al-Hesayen A, Azevedo ER, Mak S, Allard JP, Newton GE. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Am J Clin Nutr 2011; 93 (2): 332-7. [ Links ]
8. Bentley B, Lennie TA, Biddle M, Chung ML, Moser DK. Demonstration of psychometric soundness of the Dietary Sodium Restriction Questionnaire in patients whit heart failure. Heart Lung 2009; 38 (2): 121-8. [ Links ]
9. d'Almeida KSM, Souza GC, Rabelo ER. Cross-cultural adaptation into Brazilian Portuguese of the Dietary Sodium Restriction Questionnaire (DSRQ). Arq Bras Cardiol 2012; 98 (1): 70-5. [ Links ]
10. Guillemin F. Cross-cultural adaptation and validation of health status measures. Scand J Rheumatol 1995; 24 (2): 61-3. [ Links ]
11. Beaton D, Bombardier C, Guillemin F, Ferraz MB. Recommendations for the Cross-Cultural Adaptation of the DASH & Quick DASH Outcome Measures. Institute for Work & Health 2007; 1 (1): 1-4S. [ Links ]
12. Fachel JMG, Camey S. Avaliação psicométrica: a qualidade das medidas e o entendimento dos dados. In: Cunha JA. Psico-diagnóstico -V. 5ed. Artmed. Porto Alegre. 2000. [ Links ]
13. Chwalow AJ. Cross-cultural validation of existing quality of life scales. Patient Educ Couns 1995; 26 (1 Suppl. 3): 313-18. [ Links ]
15. Cortina J. What is Coefficient Alpha? An Examination of Theory and Applications. J Appl Psychol 1993; 78 (1): 98-104. [ Links ]
16. Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess 2003; 80 (1): 99-103. [ Links ]
17. León DAD. Análise Fatorial Confirmatória através dos Sofwares R e Mplus. Trabalho de Conclusão de Curso, Bacharelado em Estatística - Universidade Federal do Rio Grande do Sul; 2011. [ Links ]
18. Cornélio ME, Gallani MCBJ, Godin G, Rodrigues RCM Mendes RDR, Nadruz Junior W. Development and reliability of an instrument to measure psychosocial determinants of salt consumption among hypertensive patients. Rev Latino-Am Enfermagem 2009; 17 (5): 701-7. [ Links ]
19. Kollipara UK, Jaffer O, Amin A, Toto KH, Nelson LL, Schneider R, Makkham D, Drazner MH. Relation of Lack of Knowledge About Dietary Sodium to Hospital Readmission in Patients Whit Heart Failure. Am J Cardiol 2008; 102 (9): 121-215. [ Links ]
20. Welsh D, Marcinek R, Abshire D, Lennie TA, Biddle M, Bentley B, Moser DK. Theory-based low-sodium diet education for heart failure patients. Home Health Nurse 2010; 28 (7): 432-41. [ Links ]
21. Yehle KS, Plake KS. Self-efficacy and educational interventions in heart failure: a review of the literature. J Cardiovasc Nurs 2010; 25 (3): 175-88. [ Links ]
22. Bentley B, De Jong MJ, Moser DK, Peden Ar. Factors related to nonadherence to low sodium diet recommendations in heart failure patients. Eur J Cardiovasc Nurs 2005; 4 (4): 331-6. [ Links ]
23. Heo S, Lennie TA, Moser DK, Okoli C. Heart failure patients' perceptions on nutrition and dietary adherence. Eur J Cardiovasc Nurs 2009; 8 (5): 323-8. [ Links ]
24. Rabelo ER, Aliti GB, Goldraich L, Domingues FB, Clausell N, Rohde LE. Non-pharmacological management of patients hospitalized with heart failure at a teaching hospital. Arq Bras Cardiol 2006; 87 (3): 352-8. [ Links ]
25. Chung ML, Moser DK, Lennie TA, Worrall-Carter L, Bentley B, Trupp R, Armentano DS. Gender Differences in Adherence to the Sodium-Restricted Diet in Patients With Heart Failure. J Card Fail 2006; 12 (8): 628-34. [ Links ]
26. Gliem JA, Gliem RR. Calculating, interpreting, and reporting Cronbach s alpha reliability for Likert-type scales. Available from: http://hdl.handle.net/1805/344. [ Links ]
27. Ferketich S. Focus on psychometrics. Aspects of item analysis. Res Nurs Health 1991; 14 (2): 165-8. [ Links ]
![]() Correspondence:
Correspondence:
Eneida Rejane Rabelo da Silva
Escola de Enfermagem
Universidade Federal do Rio Grande do Sul
Rua São Manoel, 963 - Rio Branco
90620-110 Porto Alegre. RS. Brazil
E-mail: eneidarabelo@gmail.com
Recibido: 15-III-2013
1.a Revisión: 9-V-2013
Aceptado: 15-V-2013