Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Española de Enfermedades Digestivas
versão impressa ISSN 1130-0108
Rev. esp. enferm. dig. vol.103 no.7 Madrid Jul. 2011
https://dx.doi.org/10.4321/S1130-01082011000700003
Refractory iron-deficiency anemia and gluten intolerance - Response to gluten-free diet
Anemia ferropénica refractaria e intolerancia al gluten: respuesta a la dieta sin gluten
Luis Rodrigo-Sáez1, Dolores Fuentes-Álvarez1, Isabel Pérez-Martínez1, Noemí Álvarez-Mieres1, Pilar Niño García1, Ruth de-Francisco-García1, Sabino Riestra-Menéndez1, Santiago Vivas-Alegre2 and José Luis Olcoz-Goñi2
1Department of Digestive Diseases. Hospital Universitario Central de Asturias. Oviedo. Asturias, Spain.
2Unit of Digestive Diseases. Complejo Asistencial Universitario de León. Spain
ABSTRACT
Introduction: refractory iron-deficiency anemia has a multifactorial origin related to various gastrointestinal conditions, with celiac disease plus malabsorption and IBD together with isolated gluten intolerance being most common.
Objectives: to determine the prevalence of serum, genetic, and histological markers for gluten intolerance, and to analyze the response to gluten withdrawal from the diet in these patients.
Methods: a number of patients with refractory anemia were prospectively and consecutively enrolled. A protocol to measure serum (TGt-2), genetic (HLA-DQ2/DQ8), and histological markers for celiac disease was applied. All followed a gluten-free diet for a median 3.6 years. Sustained remission of anemia during follow-up was interpreted as positive response.
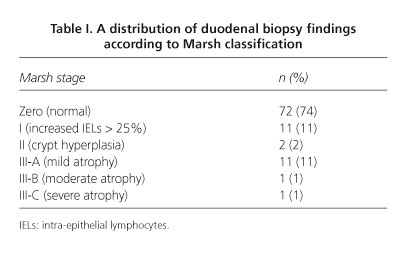
Results: ninety-eight patients (84% females) with a mean age of 54 years were studied. Anti-TGt2 antibodies were positive in 5% of cases. A total of 67 cases (68%) were haplotype HLA-DQ2 or -DQ8 (+). We found villous atrophy (Marsh III) in 13% of patients, and an inflammatory pattern (Marsh I or II) in 13%. All remaining 72 patients (74%) had no histological duodenal changes.
Age, anemia duration, number of transfusions, number of parenteral iron doses, and time on a gluten-free diet were all compared according to the presence or absence of villous atrophy and HLA-DQ2/8 positivity, and no significant differences were found for any of the analyzed variables. Response was positive in 92% of subjects.
Conclusions: celiac disease with villous atrophy is rarely a cause of refractory anemia. Gluten intolerance with no histological lesions is seen in almost 75% of patients, and therefore plays a relevant role in its development.
Key words: Refractory iron-deficiency anemia. Gluten intolerance. Gluten-free diet.
RESUMEN
Introducción: la anemia ferropénica refractaria presenta un origen multifactorial, relacionado con diversas enfermedades digestivas, siendo las más frecuentes la enfermedad celiaca con malabsorción y la EII junto con la intolerancia al gluten aislada.
Objetivos: determinar la prevalencia de marcadores serológicos, genéticos e histológicos de intolerancia al gluten y analizar la respuesta a la retirada del gluten de la dieta en estos pacientes.
Métodos: se incluyeron de forma prospectiva y consecutiva una serie de pacientes con anemia refractaria. Se les aplicó un protocolo consistente en determinación marcadores serológicos (TGt-2), genéticos (HLA-DQ2/DQ8) e histológicos de enfermedad celíaca. Todos siguieron una dieta sin gluten durante una mediana de 3,6 años. Se interpretó como respuesta positiva la desaparición mantenida de la anemia durante el seguimiento.
Resultados: se estudiaron 98 pacientes (84% mujeres) con una edad media de 54 años. Los ac. anti-TGt2 fueron positivos en el 5% de los casos. Un total de 67 casos (68%) presentaban el haplotipo HLA-DQ2 o DQ8 (+). Encontramos atrofia vellositaria (Marsh III) en el 13% de los casos y patrón inflamatorio (Marsh I o II) en el 13%. Los 72 casos restantes (74%) no presentaban alteraciones histológicas duodenales.
Se compararon la edad, el tiempo de evolución de la anemia, número de transfusiones, número de dosis de hierro parenteral y tiempo en dieta sin gluten, en función de la presencia o no de atrofia vellositaria y de la positividad para el HLA-DQ2/8, sin encontrar diferencias significativas en ninguna de las variables analizadas. La respuesta fue positiva en el 92% de los casos.
Conclusiones: la enfermedad celiaca con atrofia vellositaria es causa poco frecuente de anemia refractaria. Las formas de intolerancia al gluten sin lesión histológica asociada, representan cerca del 75% de los casos y desempeñan, por lo tanto, un papel importante en su aparición.
Palabras clave: Anemia ferropénica refractaria. Intolerancia al gluten. Dieta sin gluten.
Introduction
Overall, chronic anemia is very commonly associated with various gastrointestinal conditions. This justifies that patients with chronic anemia -at times designated anemia of obscure origin- be usually referred to a gastroenterologist in an attempt to unveil its cause and then apply the appropriate therapy.
WHO defines the presence of anemia as a reduction in blood hemoglobin levels below 13 g/dl in males and 12 g/dl in females.
According to its etiopathogenesis, chronic anemia is categorized as "central" (arregenerative) anemia when it develops as a result of defective red blood cell (RBC) production by the bone marrow, or "peripheral" (regenerative) when it is secondary to excessive RBC destruction.
Iron deficiency anemia -or ferropenic anemia- is most common, but other origins do exist. A number of gastrointestinal conditions are also usual causes (1-4).
Overall, chronic ferropenic anemia of digestive origin may be classified in two major categories: a) associated with chronic iron loss, as is the case in a number of digestive-tract benign or malignant tumors, peptic ulcer disease, NSAID use, IBD, etc.; and b) from reduced intestinal iron absorption, as occurs in celiac disease, gastric atrophy, gastrectomy, intestinal resection or bypass, etc. (5-8).
Chronic refractory iron-deficiency anemia is the type of anemia that will not respond to replacement therapy with oral iron preparations (9-12).
The present paper discusses the prevalence of classical celiac disease and of gluten intolerance in patients with chronic ferropenic anemia, as well as their response to gluten-free diet, in the absence of usual celiac disease markers.
Methods
We performed an observational, prospective study of a consecutive series of patients who attended our clinic because of refractory anemia following referral by the Internal Medicine and Hematology departments in our hospital. All patients in the series had been diagnosed based on the presence of chronic ferropenic anemia (> 6 month's standing) unresponsive to replacement therapy with oral iron preparations.
They were referred to a gastroenterology clinic for the study of small bowel conditions in the hospital, and were assessed by the same gastroenterologist (LR). They were invited to take part in a study for the screening of potential celiac disease. In all, 90% of subjects accepted and signed an informed consent. The study was approved by our hospital's ethics committee.
All patients underwent specific medical history taking and a number of laboratory tests including serum and genetic markers for CD, gastroscopy, and multiple duodenal biopsies, as well as a complete colonoscopy (through to the cecum) to rule out organic disease.
Lab tests included CBC and ESR by using an automated Cell-DYN 3500 R system (Lab. Abbott), and a complete coagulation screen using an ACL 3000 system (Lab. Menarini). Iron-deficiency anemia was diagnosed according to WHO criteria.
WBC count was considered normal for values of 4-10 x 103/L, and platelet count was considered normal for values of 130-400 x 103/L.
An extensive biochemistry panel was obtained including the following parameters: iron metabolism with sideremia, transferrin saturation index (TSI), and serum ferritin. Normal sideremia was 60-140 mcg/ml, and normal ferritin was 13-150 ng/ml. A TSI of 25-45% was considered normal.
We also obtained so-called liver function tests (LFTs) including AP, AST, ALT, GGT, and bilirubin; serum calcium, folic acid, and vitamin B-12 levels, plasma creatinine, total cholesterol (normal, 150-240 mg/dl), HDL, LDL, triglycerides, urea, glucose, total proteins, albumin, and acute-phase reagents such as CRP (C-reactive protein). Normal AST and ALT values were 1-31 U/L. All measurements were performed with a modular automated Hitachi SXA DPPP analyzer (Roche) using enzymatic or kinetic methods.
Immunoglobulin quantitation was performed using nephelometry. For serum screening, only tissue transglutaminase 2 IgA antibodies (TGt-2) were used, with measurements carried out using a commercially available ELISA kit (Phadia Diagnostics, Uppsala, Sweden). We considered it positive for values > 2 U/ml just as studies for general population screening recommend, since this threshold has a higher diagnostic sensitivity (13).
For the study of genetic susceptibility for CD both HLA-DQ2 (DQA1*0501 and DQB1*0201) and HLA-DQ8 (DQA1*0301 and DQB1*0302) markers were measured with PCR using specific sequence primers (SSPs) for DNA based on a commercially available kit designated Protrans® Domino System HLA Celiac Disease (Protrans, Ketsch, Germany).
All patients underwent a complete colonoscopy to rule out the possible existence of organic pathology or the presence of some kind of injury that could justify the origin of anemia in the colon.
All patients included in the study underwent upper digestive endoscopy with multiple duodenal biopsy taking (4-6). Samples were routinely stained with hematoxylin-eosin (H-E) and specific immunohistochemical stains for CD3 to assess the presence of intra-epithelial lymphocytes (IELs), which were counted for every one hundred epithelial cells.
Duodenal biopsies were studied by two pathologists with CD-related expertise, and classified into the following categories -stage 0 = histologically normal duodenum; stage I = increased IELs with count > 25% of epithelial cells; stage II = crypt hyperplasia and/or diffuse chronic inflammatory infiltration at the lamina propria; stage III = villous atrophy subdivided into three categories: a) mild; b) moderate; and c) severe, according to the pathological classification for CD screening as described by Marsh in 1992 (14) and modified by Oberhuber et al in 1999 (15).
All patients agreed to follow a gluten-free diet for a mean 36 ± 1.6 years (range 1-9 years), with a median of 3.6 years.
Transfusion needs and parenteral iron administration were all assessed during follow-up as well as treatment response -sustained anemia remission with no oral iron replacement therapy was considered positive.
Statistical analysis
For continuous variables a descriptive analysis was performed by estimating mean, standard deviation, and range values. For qualitative parameters percentages were used. For between-group comparisons we used Student's t-test for continuous variables in non-paired groups, and the chi-squared test for categorical variables; Fisher's exact test was used when deemed necessary. Statistical significan-ce was defined for p < 0.05.
Results
A total of 98 patients were studied. Sex distribution was 82 women (84%) vs. 16 men (16%), with a clear predominance of females and a male/female ratio of 5/1.
Mean age was 54 ± 17 years with a wide range between 22 and 90 years of age. Mean refractory ferropenic anemia duration was 13 ± 7 years, also with a wide range between 1 and 35 years.
Anti-TGt titers were positive for 5 patients only (5%), and 67 patients (68%) had a positive haplotype for HLA-DQ2 or DQ8.
Histiological findings found in duodenal biopsies and classified according to Marsh stages are represented below (Table I).
Age, anemia duration, number of transfusions, number of parenteral iron doses, and time on a gluten-free diet were all compared according to the presence or absence of villous atrophy, with no statistically significant differences regarding histology findings (Table II).
These same variables were compared between those with HLA-DQ2/8 positivity and those expressing a different allele, with no significant differences in any of the analyzed variables (Table III).
In the group with positive DQ2/8 (n = 67) those with villous atrophy (n = 12) were compared to those without it (n = 55), and no statistically significant differences were found among the above variables. Among the 83 patients without villous atrophy no differences were seen between those with a normal duodenal mucosa (Marsh 0; n = 72) and those with lymphocytic enteritis (Marsh I; n = 11).
Response to GFD was obtained for 90 patients (92%). In 5 cases it was due to complete or partial lack of compliance, or frequent dietary violations, and in 3 cases resulted from the presence of colonic (2) and gastroduodenal (1) dysplasias developed during follow-up.
Discussion
The present study reveals that gluten intolerance without associated overt celiac disease, while meeting no diagnostic criteria for CD, can originate chronic iron-deficiency anemia in three quarters of cases in a wide series of patients with refractory anemia. The most likely explanation is a disordered intestinal iron absorption, either functional or biochemical in nature (15), with no need for duodenal histology disease or villous atrophy as previously thought, which had to be found in most CD cases in agreement with classically established criteria (16).
Thus, in a recently reported study, performed on 4120 patients with ferropenic anemia, the authors observed an obscure origin in 206 (5%), 30 of which (15%) had gluten-sensitive bowel disease. Of these, 16 cases had Marsh-III lesions; 12 were Marsh-II, and 2 cases had Marsh-I. Anemia relevance was somewhat related to duodenal lesion type. As with our patients, all of them recovered from anemia by following a gluten-free diet long term (17).
The primary limitation of the present study is its observational character, as it lacks a control group for comparison of anemia response to GFD in the wide group of study subjects with normal histology versus intraepithelial lymphocytosis.
Celiac disease (CD), also known as "gluten-sensitive enteropathy", is a systemic process associated with frequent digestive involvement in the small bowel that presents with various clinical manifestations. It is a common disease with a mean prevalence around 1-2% worlwide, and affects all races in all countries; it is commonly recognized by its classical development during childhood, but may also develop -with an increasingly higher frequency- in the adult, and may be recognized and diagnosed anytime during life (18,19).
It is accompanied by a high frequency of several blood disorders, the most predominant of which is doubtless anemia. Anemia usually has a multifactorial origin and is commonly associated with deficient absorption of a number of micronutrients or trace elements, with iron being most frequently involved; however, it may also be associated with folic acid and vitamin B-12 deficiency.
CD may also be associated with other blood disorders such as leukopenia, thrombocytosis, splenic hypofunction, and occasionally malignancies, of which non-Hodgkin lymphoma and T-cell intestinal lymphoma are most common (20).
Anemia is therefore a common finding in patients with CD, and a common driver of CD diagnosis, as it may also be the only laboratory anomaly (21). This commonly occurs in patients at diagnosis, and is particularly frequent in the adult, as it is more common that celiac disease presents with atypical or pauci-symptomatic forms in this age group than in children, where abdominal manifestations are clearer and classic forms predominate with severe gastrointestinal complaints. Adult iron-deficiency anemia may be the only manifestation of CD in the absence of diarrhea (22).
The prevalence of anemia varies greatly in celiac patients at diagnosis, but ranges as wide as 15-70% of cases are reported, being higher in patients with refractory anemia (23,24). Nevertheless, the mean minimal number found in several epidemiological studies is around 5% of celiac patients before GFD onset (25).
Ferropenic anemia is a common process whose primary causes may be gathered into two major groups it is either due to chronic loss usually from lesions in any gut segment or to chronic intestinal absorption deficiencies (26-28). Iron is absorbed from the proximal portion of the small bowel, primarily the duodenum, and its complex absorption mechanism may be modified by various factors -most influential are- both from a histological and functional perspective a normal duodenal mucosa and other factors such as gastric and duodenal acidity. Anemia is characterized by low hemoglobin levels and usually microcytic and hypochromic RBCs. Patients with ferropenic anemia usually present with reduced circulating iron levels, low transferrin saturation index, and depleted body iron deposits, which translates into severely decreased serum ferritin levels (29).
Capsule endoscopy is very useful for the study of small bowel morphology, as it allows the detection of small le-sions such as angiodysplasia and little erosions or tumors that would otherwise be overlooked (30).
The measurement of circulating soluble transferrin receptor (sTfR) levels is useful to assess patients with iron-deficiency anemia, and a higher sTfR to ferritin ratio may guide CD diagnosis in children with refractory ferropenic anemia (31). Chronic iron deficiency refractory to oral iron replacement therapy may be the only CD manifestation, especially in pediatric patients. The prevalence of CD in adult patients with refractory anemia may reach up to 20% of cases.
It is also a common finding in patients with herpetiform dermatitis-like skin lesions, which is the form most frequently associated with CD.
The presence of so-called CD endoscopic markers in patients with ferropenic anemia has usually revealed a low diagnostic sensitivity, and therefore is of limited use for screening patients who will undergo duodenal biopsies. These should be undertaken even when the mucosal appearance is perfectly normal to the endoscopist. A recent study revealed that many patients undergoing upper digestive endoscopy for the study of obscure-origin ferropenic anemia have no duodenal biopsies because of the normal looks of the duodenal mucosa (32).
Ferropenic anemia is common among the general population. It often develops in younger women, and is usually attributed to increased menstrual losses; initial empiric therapy before a causal diagnosis is made is common in clinical practice. Similarly, anemia persistence after the menopause should prompt a number of studies aimed at the detection of associated CD (33).
Indeed, many patients undergo hysterectomy for hypermenorrhea or associated myomas in order to treat ferropenic anemia prior to the identification of its true origin, and anemia persists after the procedure until a correct diagnosis is ultimately reached and an appropriate therapy is indicated. Interestingly, ferropenic anemia is scarcely diagnosed in children, and is much more common among younger adults on reaching adolescence. An explanation for such discrepancy may be that hemoglobin is not routinely measured in children. What is surprising is that not all patients with gluten intolerance manifest anemia. A possible reason in some cases is that subjects are carriers of the hemochromatosis gene, C282Y or H63D (34).
In conclusion, refractory iron-deficiency anemia is common in patients with CD; even when the duodenal mucosa has normal macroscopic looks and duodenal biopsies show no apparent lesions during histology, a response to GFD may be seen. Gluten withdrawal from the diet may be an effective measure, together with the administration of oral or parenteral iron preparations, until iron deposits go back to normal and anemia is permanently solved. This is a slow, gradual process that may last one year on average, and up to 2 years for complete normalization. Prospective randomized studies of GFD versus a normal diet may ultimately bear out in a conclusive manner the efficacy of GFD in patients with refractory anemia, thus supporting the preliminary results obtained in the present paper.
References
1. Gomollón F, Gisbert JP. Anemia and digestive diseases: An update for the clinician. World J Gastroenterol 2009;15:4615-6. [ Links ]
2. Moreno Chulilla JA, Romero Colás MS, Gutiérrez Martin M. Classification of anemia for gastroenterologists. World J Gastroenterol 2009;15:4627-37. [ Links ]
3. Lanas A. Nonsteroidal anti-inflammatory drugs and cycloxigenase inhibition in the gastrointestinal tract: a trip from peptic ulcer to colon cancer. Am J Med Sci 2009;338:96-106. [ Links ]
4. Gómez Rodriguez BJ, Ortiz Moyano C, Romero Castro R, Caunedo Álvarez A, Hernández Durán MD, Hergueta Delgado P, et al. Diagnostic yield of 335 push video-enteroscopies. Rev Esp Enferm Dig 2006;98:82-92. [ Links ]
5. Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori and autoimmune gastritis. Semin Hematol 2009;46:339-50. [ Links ]
6. Raju GS, Gerson L, Das A, Lewis B. AGA Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology 2007;133:1694-6. [ Links ]
7. De la Morena F, Gisbert JP. Anemia and inflammatory bowel disease. Rev Esp Enferm Dig 2008;100:285-93. [ Links ]
8. Sharma N, Begum J, Eksteen B, Elagib A, Brookes M, Cooper BT, et al. Differential ferritin expression is associated with iron deficiency in celiac disease. Eur J Gastroenterol Hepatol 2009;21:798-804. [ Links ]
9. Clark SF. Iron deficiency anemia. Nutr Clin Pract 2008;23:128-41. [ Links ]
10. Finberg KE. Iron-refractory iron deficiency anemia. Semin Hematol 2009;46:378-86. [ Links ]
11. Oreiro MB. Iron-deficiency anemia. Treeatment. Rev Esp Enferm Dig 2009;101:70. [ Links ]
12. Lawrence LW. Refractory anemia and the myelodysplastic syndromes. Clin Lab Sci 2004;17:178-86. [ Links ]
13. Marine M, Fernández-Bañares F, Alsina M, Farré C, Cortijo M, Santaolalla R, et al. Impact of mass screening for gluten-sensitive enteropathy in working population. World J Gastroenterol 2009;15:1331-8. [ Links ]
14. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 1992;102:330-54. [ Links ]
15. Oberhüber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease. Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185-94. [ Links ]
16. Pemmaraju N, Dossopoulos T, Molitemo AR. An absorbing problem. Am J Med 2007;20:229-30. [ Links ]
17. Murray JA. Celiac disease in patients with an affected member, type 1 diabetes, iron-deficiency or osteoporosis?. Gastroenterology 2005; 128:S52-6. [ Links ]
18. Zamani F, Mohamadnejad M, Shakery R, Amiri A, Najafi S, Alimohamadi SM, et al. Gluten sensitive enteropathy in patients with iron deficiency anemia of unknown origin. World J Gastroenterol 2008; 14:7381-5. [ Links ]
19. Green PH, Jabri B. Coeliac disease. Lancet 2003;362:383-391. [ Links ]
20. Brousse N, Meijer JW. Malignant complications of coeliac disease. Best Pract Res Clin Gastroenterol 2005;19:401-412. [ Links ]
21. Unsworth DJ, Lock FJ, Harvey RF. Iron-deficiency anaemia in premenopausal women. Lancet 1999;353:1100. [ Links ]
22. Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol 1999;94:691-696. [ Links ]
23. Garrido C, Gayá J, Llompart A, Vaquero P, Sansó A, Riera J, et al. Prevalencia de enfermedad celíaca monosintomática en pacientes con anemia ferropénica. Gastroenterol y Hepatol 1997;20:172-4. [ Links ]
24. Bode S, Gudman-Hoyer E. Symptoms and haematologic features in consecutive adult celiac patients. Scand J Gastroenterol 1996;31:54-60. [ Links ]
25. Takei N, Mukai Y, Hasegawa Y, Suzukawa K, Nagata M, Noguchi M, et al. Refractory iron deficiency anemia as the primary clinical manifestation in celiac disease. Ann Hematol 2003;82:53-6. [ Links ]
26. Ackerman Z, Eliakim R, Stalnikovicz R, Rachmilewitz D. Role of small bowel biopsy in the endoscopic evaluation of adults with iron deficiency anemia. Am J Gastroenterol 1996;91:2099-102. [ Links ]
27. Andrews NC. Disorders of iron metabolism and heme synthesis. En: Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. 11th ed. Vol 1. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. p. 979-1009. [ Links ]
28. Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 2005;18:319-332. [ Links ]
29. De Caterina M, Grimaldi E, Di Pascale G, et al. The soluble transferrin receptor (sTfR)-ferritin index is a potential predictor of celiac disease in children with refractory iron deficiency anemia. Clin Chem Lab Med 2005;43:38-42. [ Links ]
30. Caunedo A, Rodriguez Téllez M, García Montes JM, Gómez Rodriguez BJ, Guerrero J, Herrerías JM Jr, et al. Usefulness of capsule endoscopy in patients with suspected small bowel disease. Rev Esp Enferm Dig 2004;96:10-21. [ Links ]
31. Economou M, Karyda S, Gombakis N, Tsatra J, Athanassiou-Metaxa M. Subclinical celiac disease in children: refractory iron deficiency as the sole presentation. J Pediatr Hematol Oncol 2004; 26:153-154. [ Links ]
32. Riestra S, Dominguez F, Fernández-Ruiz E, García-Riesco E, Nieto R, Fernández E, et al. Usefulness of duodenal biopsy during routine upper gastrointestinal endoscopy for diagnosis of celiac disease. World J Gastroenterol 2006;12:5028-32. [ Links ]
33. Ransford RA, Hayes M, Palmer M, Hall MJ. A controlled, prospective screening study of celiac disease presenting as iron deficiency anemia. J Clin Gastroenterol 2002;35:228-33. [ Links ]
34. Butterworth JR, Cooper BT, Rosenberg WM, Purkiss M, Jobson S, Hathaway M, et al. The role of hemochromatosis susceptibility gene mutations in protecting against iron deficiency in celiac disease. Gastroenterology 2002;123:444-9. [ Links ]
![]() Correspondence:
Correspondence:
Luis Rodrigo Sáez.
Servicio de Aparato Digestivo.
Hospital Universitario Central de Asturias.
C/ Celestino Villamil s/n.
33006 Oviedo, Asturias. Spain.
e-mail: lrodrigosaez@gmail.com
Received: 30-08-10.
Accepted: 10-02-11.











 texto em
texto em