Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 no.2 Madrid Abr./Jun. 2016
Osteomalacia in a young adult
Osteomalacia en un adulto joven
Alonso G.1 and Varsavsky M.2
1 Servicio de Endocrinología - "Humane Especialidades Médicas" - Río Cuarto (Argentina)
2 Servicio de Endocrinología - Hospital Italiano de Buenos Aires - Buenos Aires (Argentina)
SUMMARY
Cases of hypophosphatemic osteomalacia respond to various causes, both genetic and acquired. Some variants of mesenchymal tumors produce inappropriate amounts of fibroblast growth factor 23 (FGF-23), a mediator which induces renal phosphate loss. The biochemical picture is characterized by hypophosphatemia, decreased tubular reabsorption of phosphates, low or inappropriately normal serum calcitriol and high or unusually normal levels of FGF-23 plasma. This paraneoplastic syndrome is called tumor-induced or oncogenic osteomalacia.
There are a limited series of published cases, although it has been increasingly accepted in recent years. Diagnosis may be complex given its low incidence, the difficulties in localizing the tumors and heterogeneity in histopathologic interpretation. Complete surgical removal has healed, but there may be recurrences whereas phosphorus and calcitriol oral supplements offer alternative medical treatment.
Key words: hypophosphatemia, oncogenic osteomalacia, fibroblast growth factor 23, phosphaturic mesenchymal tumor.
RESUMEN
Los cuadros de osteomalacia hipofosfatémica responden a diversas causas genéticas y adquiridas. Algunas variantes de tumores mesenquimales producen cantidades inapropiadas de factor de crecimiento fibroblástico 23 (FGF-23), un mediador que induce una pérdida renal de fosfatos. El cuadro bioquímico se caracteriza por hipofosfatemia, disminución de la reabsorción tubular de fosfatos, niveles bajos o inapropiadamente normales de calcitriol sérico y niveles altos o inapropiadamente normales de FGF-23 plasmático. Este síndrome paraneoplásico es denominado osteomalacia tumoral u oncogénica. Existen limitadas series de casos publicadas, pero su reconocimiento es creciente en los últimos años. El diagnóstico puede ser complejo por su baja incidencia, la dificultosa localización de los tumores y la heterogeneidad en la interpretación histopatológica. La exéresis quirúrgica completa es curativa, pero puede haber recidivas y los suplementos orales de fósforo y calcitriol son alternativas de tratamiento médico.
Palabras clave: hipofosfatemia, osteomalacia oncogénica, factor de crecimiento fibroblástico 23, tumor mesenquimal fosfatúrico.
Introduction
Paraneoplastic syndromes are a set of remote effects of a tumor on different organs and systems. These are mediated by molecules with hormonal action, growth factors, cytokines, autoimmune mechanisms and other unknown factors. A rare paraneoplastic syndrome is caused by renal phosphate loss induced by inappropriate tumor secretion of fibroblast growth factor 23 (FGF-23). The condition is characterized by a generally severe hypophosphatemic osteomalacia, and termed tumor-induced osteomalacia (TIO) or oncogenic osteomalacia in the literature. In this report, a case that illustrates the diagnostic and therapeutic difficulties of this syndrome is described.
Clinical Case
A 41-year-old man at the first consultation presents a case history that started 9 years earlier with minor trauma fractures to hands and lower limbs, diffuse bone pain and progressive proximal muscle weakness. Various medical centers offered differing diagnostic possibilities, such as ankylosing spondylitis, metastatic prostate carcinoma, primary hyperparathyroidism and Paget's bone disease. He was treated in several cycles with pamidronate, zoledronate and intravenous ibandronate, oral calcium supplements, vitamin D and analgesics.
Response to this therapy was not effective, with progression of the clinical and radiological manifestations and important functional limitation. He presented no other relevant medical history or family history of metabolic bone diseases.
At the time of the first consultation, a review of previous studies showed several biochemical determinations with hypophosphatemia and high values of phosphaturia, parathyroid hormone (PTH) and total alkaline phosphatase (TAP). Remaining mineral metabolism parameters were normal. Plain radiographs showed lesions compatible with pseudo-fractures in metatarsals, metacarpals, pelvis and proximal end of the tibia and fibula. Bone scans showed multiple foci of increased uptake in long bones of the limbs, pelvis, vertebrae and costal arches. Bone densitometry showed low bone mass in the lumbar spine and femoral neck (T-score= -1.4 and -1.3, respectively). Two iliac crest biopsies were taken, directed by computed tomography (CT), without prior tetracycline marking, unspecific and insufficient for the diagnosis of Paget's bone disease.
Magnetic resonance imaging (MRI) was also carried out on the neck and lumbosacral spine, abdomen and pelvis CT scan, sestamibi parathyroid and upper endoscopy with biopsy of gastric and duodenal mucosa, all without significant findings. In Table 1 the initial results in our laboratory are summarized. No measurements could be carried out for calcitriol and serum fibroblast growth factor 23 (FGF-23).
Given this background, the diagnosis of hypophosphatemic osteomalacia was reconsidered. The patient began treatment with oral supplements of phosphorus salts (equivalent to 1,000 mg of elemental phosphorus) and calcitriol (0.50 mg daily). Clinical improvement and partial biochemical (Table 1) was observed. A positron emission tomography with fluoro-deoxyglucose (FDG-PET / CT), showing multiple hypermetabolic foci of bone location and an area of moderate uptake between the 2nd and 3rd right metatarsal (Figure 1) was requested.
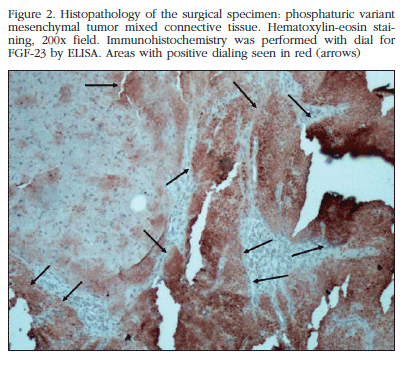
MRI showed that location in a hypointense, heterogeneous tumor lesion, lobed edges 3 by approximately 2 cm. Then resection surgery of the lesion was performed. Pathological examination reported the presence of osteoclastoides and mesenchymal cells arranged in irregular morphology varied sheets, pseudocartilaginoso stroma and prominent vascularization with hemangiopericitoide focal pattern, initially interpreted as enchondroma and in a second review as chondromyxoid fibroma. Subsequent laboratory surgery showed persistence of biochemical abnormalities (Table 1). Further MRI tests detected the presence of residual tumor. We proceeded to a second intervention to remove the residual lesion. The piece is sent to another pathologist who reported variant phosphaturic mesenchymal tumor mixed connective tissue. Immunohistochemistry using immunoperoxidase technique detected an expression of FGF-23 in large areas of the tumor (Figure 2).
The patient experienced a gradual clinical improvement, with recovery of muscle strength and progressive resolution of bone pain. Post-op control after stopping oral calcitriol phosphorus supplements indicated laboratory parameters remained within normal limits (Table 1).
Discussion
Symptoms and signs of TIO are similar to familiar hypophosphatemic osteomalacia. The main clinical manifestations in adults are bone pain, proximal muscle weakness and fractures. The clinical picture may be confused with rheumatic, oncological, psychiatric and other diseases, leading to a varying delay in the correct diagnosis.
In our patient the study presented the first manifestations at 32 years of age, resulting in numerous visits to different specialists with several different diagnoses and treatments with bone anti-resorptive for years without clinical improvement. The biochemical diagnosis is based on the finding of hypophosphatemia, hyperphosphaturia, decreased tubular reabsorption of phosphate (TRP), low or inappropriately normal serum calcitriol and inappropriately normal or high levels of plasma FGF-23 [1].
Some authors recommend the determination of the tubular threshold phosphate adjusted by glomerular filtration rate (TMP/GFR), as their values are independent of the level of plasma phosphorus and renal function, although this may vary by age and sex and has not been validated in widespread population studies [2]. Often secondary hyperparathyroidism is a physiological response to low levels of calcitriol. TSAP and the bone isoenzyme (BI) may be elevated due to increased osteoblast activity.
In the case presented here, the revision of previous analyses and our initial laboratory showed hypophosphatemia, inappropriately low TRP, high levels of TRP and TSAP. The determination of serum FGF-23 by ELISA can confirm the clinical diagnosis, although there are cases of TIO with normal serum FGF-23 [3]. FGF-23 is normally expressed by osteocytes, regulates phosphorus metabolism and vitamin D by binding to Klotho-FGF [4] receptor complex and has been linked to the pathophysiology of several entities (Table 2). At renal level it reduces renal tubular reabsorption of phosphates by decreasing expression cotransporter sodium/phosphate type 2a and 2c (NaPi-2a 2c) and inhibiting the activity of the 1α-hydroxylase kidney. These mechanisms result in hypophosphatemia, hyperphosphaturia and low levels of calcitriol.
Osteomalacia linked to tumors is usually small, benign, slow-growing and often located in the extremities, both bone and soft tissue. In our case the lesion was located in soft tissues of the plantar of foot. Cases have also been reported in paranasal sinuses, nasopharynx, brain, ovary, and pelvic column [5-7].
Sometimes the tumor is not found, and some authors refer to this situation as similar to tumor-induced osteomalacia. This has been linked to sarcomas, prostate and breast carcinomas, multiple myeloma, chronic lymphocytic leukemia and small cell carcinomas [1]. Paraneoplastic syndrome is now recognized as tumor-induced osteomalacia associated with mesenchymal tumors generally described as benign giant cell tumors, ossifying fibromas, osteoblastomas, granulomas, hemangiopericytomas and other names.
Weidner et al. proposed the term phosphaturic mesenchymal tumors, subdividing them into categories: mixed connective tissues, simile-osteoblastoma tumors, tumors simile-non-ossifying fibroma, ossifying fibroma tumors and simile-metastatic tumors [8]. The first variant represents 75% of cases in the literature identified as PMT-MCT (phosphaturic mesenchymal tumor mixed connective tissue). They are characterized by osteoclast-like giant cells, myxoid stroma or chondromyxoid, low or absent mitotic activity, ossified areas and important vascularization, with vessels of different size and morphological pattern. While it is generally benign, tumors have also been reported malignant metastatic presentations [9].
In our case there were discrepancies in the interpretation of three pathologists, and it was finally characterized as PMT-MCT. As localization methods have been proposed sinus CT, total body MRI, FDG-PET/CT, scintigraphy with labeled octreotide labeled with 111In and 99mTc scintigraphy or 201Th sestamibi.
In recent years, DOTANOC AG68-PET/CT and venous sampling dosage of FGF-23 have been included in areas where functional studies suggest suspicious injuries [10]. The FDG-PET/CT is a method of high sensitivity but low specificity, especially in patients who have many areas of pseudofractures, healing fractures or lytic areas [11].
In this case, the phosphaturic tumor was small, benign, slow-growing, was located by FDG-PET/CT and confirmed using MRI. The treatment of choice is complete surgical resection of the tumor with a wide margin, as described postsurgical recurrences [12]. When the intervention is successful the clinical and biochemical features are gradually resolved, although some of its manifestations may persist for several months. Metastasizing late recurrence is possible but rare, and pulmonary involvement has been the most commonly reported1. While particular surgery, or to an incomplete excision tumor recurrence, medical treatment is indicated with oral supplements of phosphorus salts (15-60 mg/kg per day of elemental phosphorus) and calcitriol (0.50 to 1.0 µg/day) in separate doses (4-6 times per day).
Our patient received supplements while the location and tumor excision was finalized, achieving a partial improvement of his condition. Variable results have been reported with octreotide, cinacalcet, radiofrequency ablation and intratumoral injection of ethanol.
Using monoclonal antibodies is a novel approach that disrupts the interaction of FGF-23 with its receptor [13]. The series of published cases highlight the main features of TIO: diagnostic delay, the difficult location of tumors, predominantly in the lower limbs, healing after complete removal and the possibility of relapse [14]. Less than 400 TIO cases have been reported, mostly in recent years, reflecting its low incidence and difficulties in identification.
The case presented illustrates the difficulty and delay in diagnosis, persistently incomplete resection of phosphaturic tumor and discrepancies in interpretation.
Acknowledgements
We would like to thank Dr. Beatriz Oliveri for her valuable contribution in solving the case, and Dr. Rosa Moysés for help in immunostaining for FGF-23 on biopsy.
![]() Correspondence:
Correspondence:
Mariela Varsavsky
Juan D. Perón 4190
C1181ACH - Buenos Aires (Argentina)
e-mail: mariela.varsavsky@hospitalitaliano.org.ar
Date of receipt: 17/05/2016
Date of acceptance: 14/07/2016
Bibliography
1. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53-77. [ Links ]
2. Barth JH, Jones RG, Payne RB. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem. 2000;37:79-81. [ Links ]
3. Amblee A, Uy J, Senseng C, Hart P. Tumor-induced osteomalacia with normal systemic fibroblast growth factor-23 level. Clin Kidney J. 2014;7(2):186-9. [ Links ]
4. Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611-9. [ Links ]
5. Fathalla H, Cusimano M, Di Ieva A, Karamchandani J, Fung R, Kovacs K. Osteomalacia-Inducing Tumors of the Brain: A Case Report, Review and a Hypothesis. World Neurosurg. 2015;84(1):189.e1-5. [ Links ]
6. Lin HA, Shih SR, Tseng YT, Chen CH, Chiu WY, Hsu CY, et al. Ovarian cancer-related hypophosphatemic osteomalacia. A case report. J Clin Endocrinol Metab. 2014;99(12):4403-7. [ Links ]
7. Meng T, Zhou W, Li B, Yin H, Li Z, Zhou L. En bloc resection for treatment of tumor-induced osteomalacia: a case presentation and a systematic review. World J Surg Oncol. 2015;13(1):176-82. [ Links ]
8. Weidner N. Review and update: oncogenic osteomalacia-rickets. Ultrastruct Pathol. 1991;15:317-33. [ Links ]
9. Ogose A, Hotta T, Emura I, Hatano H, Inoue Y, Umezu H, et al. Recurrent malignant variant of phosphaturic mesenchymal tumor with oncogenic osteomalacia. Skeletal Radiol. 2001;30:99-103. [ Links ]
10. Fukumoto S. Diagnostic Modalities for FGF23-Producing Tumors in Patients with Tumor-Induced Osteomalacia. Endocrinol Metab (Seoul). 2014;29(2):136-43. [ Links ]
11. Hesse E, Moessinger E, Rosenthal H, Laenger F, Brabant G, Petrich T, et al. Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res. 2007;22:158-62. [ Links ]
12. Sun ZJ, Jin J, Qiu GX, Gao P, Liu Y. Surgical treatment of tumor-induced osteomalacia: a retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord. 2015;26:16-23. [ Links ]
13. Kinoshita Y, Fukumoto S. Anti-FGF23 antibody therapy for patients with tumor-induced osteomalacia. Clin Calcium. 2014;24(8):1217-22. [ Links ]
14. Mastaglia S, Somoza J, González D, Oliveri B. Osteomalacia tumoral. Actualizaciones en Osteología. 2013;9:194-202. [ Links ]











 texto em
texto em