INTRODUCTION
Liposomes are utilized as transporters for both lipophilic and water solvent molecules. Hydrophilic substances are encapsulated in the interior aqueous portions whereas lipophilic substances are entrapped within lipid bilayers. Liposomes as drug delivery frameworks plays a vital role to enhance their therapeutic effect, decrease adverse effects and improve the viability of medications for the treatment of illnesses.1 Liposomes are as of now increasingly involved in the dermatology, antibody adjuvant, infective ailment, immunology, eye issue, and in tumor treatment. Various improvements have been utilized to target liposomes which lead to drug accumulation at disease sites and reduced distribution to sensitive tissues. Liposomes with enhanced drug delivery to disease locations, by the ability of long circulation residence times, are now achieving clinical acceptance.2) Release rate of different types of drug molecules from liposomes is dependent on the type of drug applied.2 Cholesterol may be included to improve bilayer characteristics of vesicles, increase microviscosity of the bilayers, reduce the permeability of the membrane to water-soluble molecules, stabilize the membrane and increase the rigidity of the vesicles.35- FU is most often prescribed for actinic keratoses and skin warts.2,3 It destroys sun-damaged skin cells so the skin appears smoother and more youthful. It works best on face and scalp, and is less effective on other areas. TTN enhances the effect of 5- FU by peeling off the top layer of skin. It reduces the time required for the course of 5- FU treatment.3
The present study was planned to formulate and evaluate a new combination of 5- FU and TTN for topical administration. The main aim of the study is to attain effective drug concentration at the intended site of action for a sufficient period of time to elicit the response.
MATERIALS
TTN and 5- FU were kindly provided by Curetech Skincare [Baddi, Himachal Pradesh] and Shalaks pharmaceuticals [New Delhi] respectively. Cholesterol and Soy Lecithin were purchased from the Central drug house [New Delhi]. Cellophane membrane (molecular weight cut off 12,000-14,000) was purchased from Qualigens Fine Chemicals [Mumbai, India]. Ethanol and all other chemicals used were of analytical grade.
METHODS
Experimental design
A 32 randomized full factorial design was adopted to optimize the variables. In the design two factors were evaluated, each at 3 levels and experimental trials were at all nine possible combinations using Design Expert Software 10 (State-Ease, inc., Minneapolis, USA). In the present investigation, the concentration of TTN (X1) and concentration of phospholipid (X2) were selected as independent variables. The entrapment efficiency (EE; R1), and cumulative drug release (% CDR; R2) were selected as dependent variables (Table 1). The polynomial equation was generated for the dependent variables. The value of p<0.05 was considered to be significant.4,5
Table 1: 32 Factorial design for preparation of liposomal formulations
| Formulation Code# | Independent Variables | Dependent Variables | Zeta Potential (mV) | Particle size (nm) | |
|---|---|---|---|---|---|
| X1* | X2$ | ||||
| F1 | -1 | -1 | -19.14±2.12 | 112±2.76 | |
| F2 | 0 | -1 | -19.99±1.98 | 100±2.34 | |
| F3 | +1 | -1 | -20.10±2.01 | 148±2.10 | |
| F4 | -1 | 0 | %EE (R1) | -24.34±1.87 | 198±1.99 |
| F5 | 0 | 0 | %DR (R2) | -19.89±2.87 | 200±1.59 |
| F6 | +1 | 0 | -21.42 ±1.34 | 167±2.37 | |
| F7 | -1 | +1 | -24.32±1.23 | 196±2.02 | |
| F8 | 0 | +1 | -23.99±1.87 | 182±2.88 | |
| F9 | +1 | +1 | -25.61±2.03 | 191±2.98 | |
*Concentration of TTN,
$Concentration of phospholipid,
#All batches contained 500 mg of 5- FU, 4mg/ml cholesterol.Coded values (-1, 0, +1); Actual values (mg/ml) (X1= 0.25, 0.75, 1.0, X2= 20, 40, 60)
Formulation of drug loaded liposomes
Drug loaded liposomes were prepared by a modified ethanol injection method.4) Required amounts of phospholipids (20, 40, 60 mg/ml) and cholesterol (4 mg/ml) were dissolved in ethanol and different concentrations of TTN (0.25, 0.75 and 1.0 % w/v) was added to the organic phase individually. Resulting organic phase was injected by means of a syringe pump to aqueous phase (500 mg of 5-FUwas added to the aqueous phase) under magnetic stirring at 45± 2 °C. Spontaneous liposome formation occurred as soon as the ethanolic solution was in contact with the aqueous phase. Liposome suspension was then kept under stirring for 1h at room temperature to remove the traces of solvent. The unloaded drug was removed by ultracentrifugation of liposome suspension (Beckman, Miami, Florida, USA) at 60,000 rpm for 1 hour and stored at 4°C.4,5
Evaluation of liposomes
Morphological study by transmission electron microscopy (TEM)
Liposome suspensions were imaged by using TEM (Philips CM120; Eindhoven, The Netherlands). A drop of the liposome suspension was placed onto a carbon-coated copper grid, forming a thin liquid film. The films were negatively stained with 2% phosphotungstic acid solution for 1 minute. The excess of phosphotungstic solution was removed with a filter paper and stained samples were characterized by using an accelerating voltage of 80 kV.6,7
Zeta potential study
The particle size and the zeta potential of liposomes were determined by dynamic light scattering (DLS), using the Zetasizer (Malvern instruments, UK). Each sample was measured three times, after which the mean of the values was calculated. The measurement was performed at 25°C after an appropriate dilution with distilled water.10-11
Entrapment efficiency
5ml of liposome formulation was taken and transferred to a 100 ml volumetric flask containing 25 ml of phosphate buffer (skin pH 6.8), then sonicated using an ultrasound bath for few minutes and filtered through a 0.45μm membrane filter. The filtrate was finally diluted with phosphate buffer (pH 6.8) and absorbance was recorded by Shimadzu 1700 UV visible spectrophotometer at 266 and 340 nm respectively for5- FU and TTN.7
In vitro drug reléase
In-vitro drug release study of liposomal formulations was performed using franz diffusion cells with diffusional area of 0.75 cm2. An egg membrane was placed between donor and receptor compartments. The receptor compartment contained phosphate buffer pH 6.8 was continuously stirred by magnetic bead and maintained at temperatureof 37 ± 1ºC. One ml liposomal suspension was loaded on the donor compartment. The drug concentrations in aliquot were withdrawn at different time intervals and analyzed at 266 nm and340 nm against appropriate blank for 5-FU and TTN respectively, using UV -Vis spectrophotometer.3 4-5) The kinetics of drug release from liposomes was determined by fitting the appropriate drug release data to zero order, first order, Higuchi equation, Hixson-Crowell equation and the Korsemeyer-Peppas model.9
Optimization of liposome using experimental design
Constraints, like maximizing entrapment efficiency and % drug release at the end of 8 hours as well as minimizing the particle size, were set as a goal to select the optimized formulation using Design expert software version 10 (Stat-Ease, Inc., Minneapolis, USA).
Histopathological evaluation of optimized liposome
A section of goatskin was harvested from local commercial supplier to evaluates histopathological condition in the presence of F9 liposomes. Harvested skin was shaved and placed on a Franz diffusion cell filled with phosphate buffer (pH 6.8 ± 0.10).Test group was treated with F9, while control group was treated with blank liposome for about eight hours. Exposed test and control tissues were fixed in 10% v/v neutral buffered formalin (pH 6.6) and were routinely processed in paraffin. Tissue sections of ~7 μm were cut on a glass slide and stained with hematoxylin-eosin. These sections were examined under light microscope to detect tissue damage caused by the formulations during the permeation studies.
RESULTS AND DISCUSSION
Evaluation of prepared liposomes
Negative-strain TEM images showed that liposomes obtained were spherical shaped, which could have an impact on drug-release. The particle size of drug-loaded liposomes was found to be 100 - 200 nm (Table 1). No drug crystals were visible in TEM-images, regardless of the preparation technique or the loaded drug (Figure 1).12) All liposomes were negatively charged and zeta-potential values varied between -19.14 and -25.61 mV (Table 1), which is considered as an optimal potential for assuring particle stability. It was observed that £ potential of prepared liposomes has sufficient charge to inhibit aggregation of vesicles.13) The average percent drug entrapment efficiency of the nine formulations ranges from 28.57 to 72.86% and 37.88 to 69.70% of 5- FU and TTN respectively, where the Formulation F9 showed a maximum drug entrapment of 72.86% and 69.70% for 5- FU and TTN respectively.
In vitro Release Study of Liposomes
The release profiles of liposomes of entire formulations were shown in Figure 2. As the concentration of phospholipid was increased from 20 to 60 mg/ml (Figure 2), using the same cholesterol percentage with respect to phospholipid amount, the cumulative released amount of drugs decreased. It has been stated that the release of lipophilic agents from liposomes is delayed because of their location within the lipid bilayers.10) From the drug release profile of formulations F2, F5, F8 it was observed that drug release was found to be increased within 2 hrs and very less amount of drug was left for further release. Release profile of formulation F9 showed that almost entire drug was released within 7hrs. The higher amount of phospholipid in F9 leads to a time-dependent increase in diffusion coefficient is a highly lipophilic drug, should be entrapped within the phospholipid bilayers. Hence, the release mechanism involves slow diffusion through the liposome wall. No sudden release occurred during the release study, indicating that no liposome disintegration had taken place. In vitro dissolution studies showed that as the concentration of phospholipid was increased, drug release rate was decreased. Dissolution profiles of formulations F1 to F6 were not good because high amount of drug release (30.6 to 67.42%) at 2 h. The results of drug release profile of the F9 showed the release of 32.48% of drug during initial 2hrs. While within the first 4 h 61.1% of drug was released and the remaining drug was released during last 4 hrs. Model with the highest correlation coefficient (r2) was judged to be a more appropriate model for the dissolution data. According to the result obtained by release kinetics in case of 5- FU formulations F1, F3, F4, F5, F7, and F8 followed the korsemeyer- peppas model, F2&F6 Hixson model, and F9 zero order kinetics. In case of TTN F1, F3, F5,F6, F8&F9 followed korsemeyer- peppas model, F2&F7 zero and F4 Higuchi kinetics.16
Optimization of Liposome Formulation Using 32 Full Factorial Design
A 32 full factorial design was constructed to study the effect of TTN concentration (X1) and phospholipid concentration (X2) on the drug release from liposomal preparations. The percentage entrapment efficiency at pH 6.8 (R1) and percentage drug release (R2) was selected as dependent variables. The main effects (X1 and X2) represent the average result of changing one factor at a time from its low to high value. The statistical model incorporating interactive and polynomial terms was utilized to evaluate the responses. The interaction terms (X1, X2) showed how the response changes when 2 factors are changed simultaneously. The full Equation (equation containing only statistically significant terms) is then used for drawing plots to visualize the impact of changing variables at a glance. The optimum point may be identified from the plot.
The factorial equation for R1 (Eq 1) and R2 (Eq 2) was found to be:
The coefficient of X1 and X2 was found to be positive indicated that predicted values could be obtained when the concentration of TTN and phospholipid increased. The p value for variable X1 and X2 was 0.0231 and 0.0112 respectively (P<0.0500) indicated that both independent variables showed a significant effect on dependent variable i.e. R1 and R2. The prediction error in the response parameters ranged between 0.47 and 0.79% to the value of the absolute error of 0.90±0.70%. The low values of error indicate the high prognostic ability of factorial equation and counter plot methodology. “Adeq Precision” measures the signal to noise ratio. A ratio greater than 4 is desirable. The ratio of 7.56 indicates an adequate signal. Thus, this model can be used to navigate the design space6. From the plot, the spread of points on the right side of the graph (where X1 is low) is larger than the spread between the points at the left side of the graph where X1 is high. In other words, the effect of X2 is less significant where X1 is high (Figure 3(A)).17,18 Therefore, at a very high X1 value, the effect of phospholipid concentration can be significantly reduced, thus reducing the R1 and R2. Ramps (Figure 3(B)) indicated that response R1 increases with a decrease in X1 and X2.19 Higher X1 and X2 reduced response R1.Ramps report clearly supported optimized formulation. After generating the polynomial equation for the dependent and independent variables, the combination was optimized for responses.20) Formulations with high X1 and X2 showed highest desirability factor of 1.00 with highest R1 and R2 was selected as optimized formulation (Figure 3(C)). The Response Surface linear model generated for X1 and X2 was found to be significant with an F-value of 2.50 and 6.80 (P<0.0500) respectively.
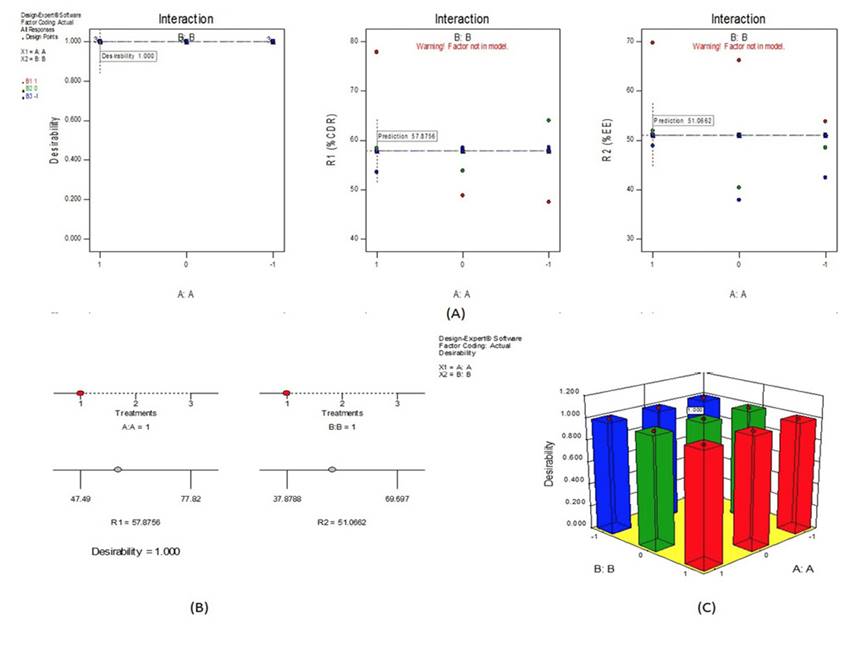
Figure 3: Graphs showing experimental design (A) Interaction of R1 and R2; (B) Ramps for Desirability; (C) 3D graph for optimization of independent variables
The histopathology results demonstrated the safety profile of developed F9 liposomes (Figure 4). The developed liposome F9 revealed no signs of damage to epithelial cells of goatskin.
CONCLUSION
In this study, liposomes containing 5- FU and TTN were prepared with different proportions of TTN and phospholipid using modified injection method. Liposomes composed of 1.0 mg/ml of TTN and 60 mg/ml phospholipid presented higher % entrapment efficiency and 30 days stability. Particle size of liposomes remained smaller than 200 nm, making them suitable for topical application. Results suggested that liposomes gradually release 5- FU and TTN and found to be topically safe as tested by histological evaluation. Prepared liposomes containing 5-FU and TTN may be successfully used for treatment of skin warts with high patient compliance.


















