Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos de Zootecnia
versión On-line ISSN 1885-4494versión impresa ISSN 0004-0592
Arch. zootec. vol.60 no.232 Córdoba dic. 2011
https://dx.doi.org/10.4321/S0004-05922011000400035
Performance of Carassius auratus with different food strategies in water recirculation system
Desempenho de Carassius auratus com diferentes estratégias alimentares em sistema de recirculação de água
Moreira, R.L.1*, Da Costa, J.M.1, Teixeira, E.G.1, Moreira, A.G.L.1, De Moura, P.S.1, Rocha, R.S.2 and Vieira, R.H.S.F.2
1Universidade Federal do Ceará (UFC). Centro de Ciências Agrárias. Departamento de Engenharia de Pesca. Campus do Pici. Fortaleza, CE. Brazil. *ricardolafaiete@hotmail.com
2Instituto de Ciências do Mar. LABOMAR/UFC. Fortaleza, Ceará. Brazil.
SUMMARY
The efficiency of a recirculation system for the cultivation of C. auratus, fed with natural and artificial diets was evaluated. The experiment consisted of four treatments with four replicates. The first two treatments used recirculation system and the last two static system. In the first and fourth treatment, animals were fed with brine shrimp biomass (50% CP), while in the second and third treatments a commercial flake ration (42% CP) was used. At the end of cultivation (60 days), water samples were taken from all experimental units and subjected to standard plate count for determining the density of mesophilic bacteria. Final weight (g), final length (cm), specific growth rate (% day -1) and weight gain (%) were significantly different (p<0.05) among tested diets, but the culture systems did not affect fish performance. There was significant difference (p<0.05) between systems for total ammonia and nitrite. Animals fed with brine shrimp biomass acquired more intense and brighter colors than those fed only with commercial flake food. The values of standard plate count of mesophilic bacteria ranged from 7.0 × 103 to 1.1 × 104 CFU.ml-1. In this study we observed that C. auratus is a species tolerant to adverse water conditions, perhaps with increased stock densities, the recirculation system could play some positive role on the zootechnical performance of cultured animals.
Key words: Growth. Goldfish. Culture. Diets.
RESUMO
A eficiência de um sistema de recirculação para o cultivo de C. auratus, alimentados com dieta natural e artificial foi avaliada. O experimento consistiu de quatro tratamentos com quatro repetições. Os dois primeiros tratamentos utilizaram sistemas de recirculação e os dois últimos sistemas estáticos. No primeiro e quarto tratamento, os animais foram alimentados com biomassa de artêmia (PB 50%), enquanto nos tratamentos dois e três, com ração comercial em flocos (42% PB). Ao final do cultivo (60 dias), amostras de água foram coletadas em todas as unidades experimentais e submetidos a contagem padrão em placas para determinação da densidade de bactérias mesófilas. O peso final (g), comprimento final (cm), taxa de crescimento específico (% dia-1) e ganho de peso (%) foram significativamente diferentes (p<0,05) entre as dietas testadas, mas os sistemas de cultivo não afetaram o desempenho dos peixes. Houve diferença significativa (p<0,05) entre os sistemas para os níveis de amônia total e nitrito. Os animais alimentados com biomassa artêmia adquiriram cor e brilho mais intensos do que aqueles alimentados apenas com ração comercial em flocos. Os valores de contagem padrão em placas de bactérias mesófilas variaram de 7,0 × 103 para 1,1 × 104 CFU.ml-1. Neste estudo, observou-se que C. auratus é uma espécie tolerante a condições adversas de água, talvez com maior densidade de estocagem, o sistema de recirculação poderia desempenhar papel positivo sobre o desempenho zootécnico dos animais cultivados.
Palavras chave: Crescimento. Peixe dourado. Cultivo. Dietas.
Introduction
Approximately 4000 freshwater ornamental fish species and 1400 marine fish species are sold per year around the world (Whittington and Chong, 2007), consolidating the aquariophily as the second biggest hobby in the world. Production of animals for the aquarium hobbyist trade is a rapidly growing sector of the aquacultural industry, and it will continue to grow in importance as restrictions on collecting animals from the wild are being placed worldwide. Presently, approximately 90% of freshwater fish traded in the hobbyist industry are captively cultured, however for marine ornamentals, the reverse is true as only for a handful of species that are produced via aquaculture (Tlusty, 2001). The Japanese fish, C. auratus, also known as goldfish, is one of the most popular ornamental species in the world due to its varieties with attractive body shape and skin color (Zhou et al., 2001). Was described by Linnaeus (1758) on the basis of an orange coloured specimen with trilobed caudal fin. It is generally considered to represent a domestic form of the silver Prussian carp, C. gibelio (Gentry et al., 2004). Its natural occurence range from northern Europe to sotheast Asia. As target for aquariophily, it has been widely used for aquaculture practice in the recent 30 years in China, and its production has reached 2 billion kilograms annually. Growth rates for this species in the wild are around 13 cm per year and animals rarely exceed 35 cm, depending on water temperature, stocking density and especially the food type they are feed. It is well known that in addition to body shape, and fins size, skin pigmentation (derived from the deposition of carotenoids in their tissues) is one of the most important quality criteria to set the market value of ornamental fish (Paripatananont et al., 1999; Lovell, 2000; Gouveia et al., 2003).
The industrial development of freshwater ornamental fish culture has been hampered by the lack of suitable live feeds for feeding the fish at the various production stages. The rotifers are an ideal starter feed for dwarf gourami (Colisa lalia), a typical freshwater ornamental fish species with larvae that are too small to ingest artemia nauplii or moina at its first feeding (Lim et al., 2003). Ornamental fishes have traditionally been fed live food, which in many cases can be nutritionally deficient, and act as a source of diseases (parasitic, bacterial and viral). Sales and Janssens (2003), investigated some nutritional requirements (protein and minerals) for growing freshwater ornamental species (live-bearers) with emphasis on the provision of live feed during the early stages of life. Protein requirements varied from 30% for growing omnivorous goldfish (C. auratus) to 50% for carnivorous discus (Symphysodon aequifasciata). Ingredients from plant proteins (e.g., Spirulina, soybean and alfalfa meals) and fibre are incorporated into diets for goldfish, koi and herbivorous fishes (e.g., certain catfishes and chichlid species) (Chapman, 2000). Bandyopadhyay et al. (2005) feeding juvenile goldfish obtained results better when fed protein levels the 42.53%.
Artemia is widely used in countries that practice commercial aquaculture due to its easiness of use or due its nutritional properties which are essential to many aquatic species during some stages of their life cycle (Barros and Valenti, 2003). This microcrustacean is among the most used foods for ornamental fish, provided either live (nauplius) or frozen (adult). Even after decades of use, it does not have a full replacement yet, and due to the fact that most of its production came from collection in the wild, it makes them a very expensive item (Joshi and Vartak, 1999). Another problem regarding the use of brine shrimp biomass as food is the high input of organic matter into the aquaculture system, which may favor bacterial growth. The knowledge of microbial diversity in the culture water is essential for the monitoring of fish health quality (Schalch and Moraes, 2005). Several bacterial general have been associated with diseases in fish, oftenly originating from the given food (Ju et al., 2007). Bacterial disease is the most common infectious problem of ornamental fishes. Collectively, only water quality problems exceed bacterial diseases in the area of pet fish morbidity and mortality. The majority of bacterial infections are caused by gram-negative organisms including the following pathogenic genera: Aeromonas, Citrobacter, Edwardsiella, Flavobacterium (Flexibacter), Mycobacterium, Pseudomonas, and Vibrio. Streptococcus, a gram-positive genus, has been shown to cause disease in ornamental fishes (Lewbart, 2001).
The development of efficient culture systems that preserve water quality is higly needed. Therefore, the use of closed of recirculating water systems is becoming one of the alternatives for aquaculture (Colt et al., 2006; Gutierrez-Wing and Malone, 2006). Marengoni et al. (2010), using recirculating water systems and cages for the culture of red tilapia. Have concluded that the application of probiotics in feed does not justify its effectiveness as growth promoter. Halachmi (2006) developed a simulation model for determining the optimal layout and management regime for ornamental fish recirculating aquaculture system. Simulation experiments facilitate the joint evaluation of fish biology, equipment, management practices, farm routine and layout and can highlight potential design options before the system is built. The addition of one reproduction cycle per year reduced the maximum biomass load from 8 to 5 ton. The addition of two reproductions per year would enable the system to process an additional 1 million fingerlings per year, elevating sales by 60% without changing the biomass load. For those reasons, this study intended to monitor growth performance of goldfish C. auratus cultured under water recirculating system, fed with natural and artificial diets and also to quantify the total mesophilic bacteria present in the culture water.
Materials and methods
Experiments were carried out in sixteen polyethylene tanks (80 l), with completely randomized design, subjected to four treatments with four replicates each: water recirculating system, fed artemia (RA), water recirculating systems with commercial ration (RR), static water fed artemia (SA) and static water fed commercial ration (SR), being two treatments using water recirculation and two using static water system. Treatments RA and RR used a 1000 l/hour filtering and pumping system equipped with mechanical (glass wool), biological (bacterial biofilm adhered to 3 cm in diameter granite rocks) and chemical (activated coal) filtration. For the static water systems a constant aeration was provided by a portable airpump connected to airstones. Initial stock density in all treatments was ten animals per tank. Frozen brine shrimp biomass was used as natural food source, while the commercial diet was composed by textured soy protein, fish meal, wheat flour, fresh shrimp, corn meal, seaweed meal, dehydrated carrot, dehydrated spinach, yeast, soybean oil, Spirulina, prebiotic additive, salt, additive enzyme, vitamin mineral premix, astaxanthin, natural dyes, organic minerals chelated stabilized vitamin C and antioxidant, according to the information provided by the manufacturer. Both tested diets were offered at 8% of the total biomass, in two meals per day, six day a week. Nutritional values of diets are shown in table I. Biometrics occurred every two weeks, zootechnical performances were evaluated regarding average weight growth, total length growth, weight gain (%), specific growth rate (%) and survival rate, according to the following formulas:
Average weight growth (g) = final biomass/number of survivors;
Total length growth (cm) = final lenght - initial length;
Weight gain (%) = (final weight - initial weight /initial weight) x 100;
Specific growth rate (% day-1) = (final weight initial weight/experimental period) x 100;
Survival rate (%) = (final number of animals /initial number of animals) x 100.
At every biometrics, animals were individually photographed with a digital camera Cyber-Shot SonyTM, 6.0 megapixels, for morphometric characterization (fins, and body color and shape). The physicalchemical water parameters oxygen (mg.l-1), pH, nitrite (NO2), general hardness and carbonate (KH and GH) and carbon dioxide (CO2) were measured weekly using commercial colorimetric tests (ALCONTM), while water temperature (oC) was measured using a digital thermometer.
Water samples were collected from all treatment at the end of the experiment and stirred at room temperature to promote the suspension of the material. 1 ml aliquots were taken with an automatic pipettor and diluted into 9 ml of saline solution 0.85%. This dilution was corresponding to the dilution of 10-1. Aliquots were successively removed and diluted at 1 ml to 9 ml until the dilution reached 10-4. From each dilution, as well as from the pure sample, a 1 ml aliquot was removed and placed in a petri dish containing Plate Count Agar culture medium (PCA) superimposed on the inoculum by the Pour Plate method. Plates were then incubated at 35oC/48 h in bacteriological incubator (Downes and Ito, 2001). After a 24 h period, plate counts were made using a colony counter model PHOENIX EC550A and revealed growth between 25 and 250 colony forming units (CFU). The resulting Standard Plate Counting was calculated by the expression: inverse of CFU x dilution factor, and expressed as CFU /ml according to Downes and Ito (2001). Those colonies that showed no growth in the stipulated range had their scores estimated. Zootechnical performance of fish and physicalchemical water parameters data were subjected to analysis of variance with one factor (ANOVA) and subsequently to the independent T test for means. Tests were performed at 5% level of statistical significance, using the statistical function of the software ORIGIN 5.0. An angular transformer (arc-sine square root) was used to homogenize the variances of survival rates values, although these values are presented in its original form. Microbiological analysis data from each treatment was submitted to ANOVA (α= 5%) using the Software Statistica version 7.0, to compare the results. In cases were homoscedasticity was not observed, data were logarithmized.
Results and discussion
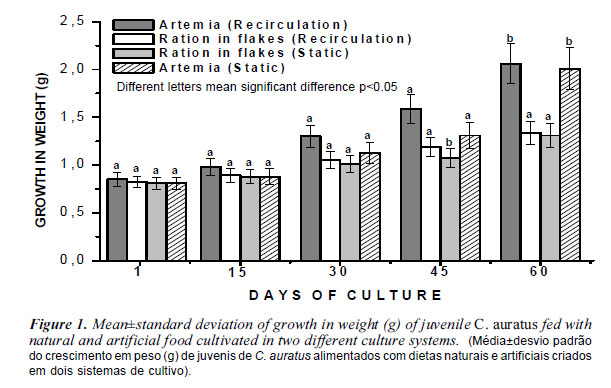
Zootechnical performance in the two treatments that used artemia as food source (RA and SA) presented superior results (p<0.05) than those treatments that used commercial food, concerning average weight growth, total length growth, weight gain, specific growth rate (figure 1 and 2, table II), regardless the culture system used. The chemical composition of the basic adult artemia is protein (65%), lipid (13.2%), carbohydrate (3%) and ash (4.8%). This microcrustacea is considered one of the most complete items that meet the nutritional requirements of fish and shrimp, being adopted as the standard food in commercial hatcheries (Sorgeloos et al., 1998 and 2001). It is a natural food rich in proteins, lipids, and especially polyunsaturated fatty acids (PUFA) and highly unsaturated (HUFA) (Hand and Podrabsky, 2000), and provides other basic nutrients for growth of aquatic animals. Goldfishes are usually grown in eutrophic ponds in which Chlorophyta and cyanobacteria are the dominant flora. As these food sources are rich in carotenoids, fish cultured in such environments stain quite prominent. However, the relative ontogenetic stage of the fish being fed can significantly affect the protein level required in their food. A high requirement level for protein (53%) was found for goldfish (C. auratus) larvae, in comparison to 29% for juvenile fish (Sales and Janssens, 2003). Nevertheless, this culture condition could not be performed in intensive systems, the sources of carotenoids must be added in the diets of fish. Lochmann and Phillips (1994) determined the protein levels needed to optimize the weight gain, feed efficiency, survival and protein efficiency ratio (PER) for juvenile C. auratus. The best results were in fish fed diets containing 28.9% protein values declined PER in fish fed diets containing more than 28.9% crude protein.
The survival rates more were achieved to treatments that used water recirculation system, fed artemia and water static system, fed ration, but were not significantly different between all tested treatments (p>0.05). Survival was independent of any diet. Gouveia et al. (2003), fed Carassius auratus and three varieties of Koi carp with dietary supplementation of carotenoids and algal biomass (Chlorella vulgaris, Haematococcus pluvialis and arthrospira maximum) using a diet containing synthetic astaxanthin and a control diet without dye for comparison. For Koi carp C. vulgaris had higher amounts of carotenoids. For goldfish, the best results were achieved with C. vulgaris and H. pluvialis. Hu et al. (2008), partially replacing fish meal by poultry byproduct meal, meat and bone meal and blood meal in practical diets for carp C. auratus gibelio. These results demonstrated that the combination of ingredients can replace up to 6% levels of fishmeal in practical diets for this species. Xue and Cui (2001) studied the effects of stimulants in the diet of carp in diets with or without the substitution of fish meal by meal and bones. Animals fed the diet containing fish meal had better results than those fed the diet containing meat and bone. The squid extract had the strongest stimulating effect among all the attractants tested. Yanar et al. (2008) evaluated the effects of diets with alfalfa feeding of adult goldfish (C. auratus). At the end of the experiment was verified skin pigmentation, growth, feed efficiency and survival. The study results showed that alfalfa can be successfully used as an alternative source of natural carotenoids for this species.
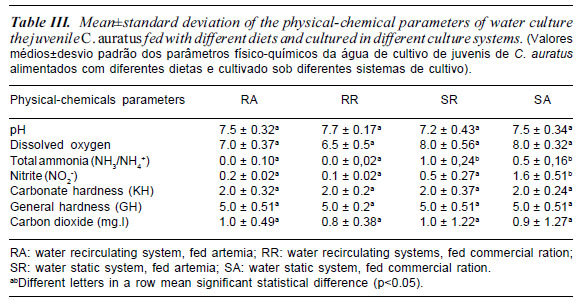
In the recirculation system the levels of total ammonia and nitrite were significantly lower than in static water system (p<0.05) as shown in table III. In recent years there has been a growing concern about the impacts caused by aquaculture operations, mainly due to the effluent discharge into the environment. Systems involving recircuation of culture water have been considere viable alternatives for minimizing environmental impacts (Buschmann et al., 1996; Naylor et al., 2000, Johnson et al., 2004). The results of the present study indicated that the different culture systems (static and recirculation) had no effect on the zootechnical performance of cultured fish. However, water recirculation systems, become a viable alternative when the associated energy costs, because the constant water flow keeps water temperature stable (minimizing the use of thermostats) and adequate oxygen levels in the water, decreasing the need for artificial aeration. It has been estimated that the production of ornamental fish under recirculation systems saves an average $0.96 for the energy per kilogram of fish produced (Greiner and Timmons, 1998). The need to produce fish all year around has been a major factor encouraging the development of recirculation systems worldwide (Gutierrez-Wing and Malone, 2006). In the current paper, high nitrogen levels found in the static system did not interfere on the zootechnical performance of cultured fish. Juvenile and adult C. auratus are tolerant to adverse environmental conditions of cultivation (Kestemont, 1995). The same author affirm that goldfish larvae require specific requirements of the physical, chemical and biological water to enhance their survival and maximize growth.
The values of the standard plate count (SPC) of mesophilic bacteria were higher than 3.0 .103 CFU/ ml for the recirculation system with natural food (RA), higher than 2.5 .103 CFU/ ml for recirculation systems with artificial food (RR), higher than 5.5 .103 CFU/ ml for static with natural food (SA) and higher tham 4.0 .103 CFU/ ml for water static with artificial food (SR) as shown in and table IV. Natural food represents a significant portion of the nutrients that are metabolized and converted into biomass for the cultured organisms (Sales and Janssens, 2003). In contrast, these can be vehicles of many microorganisms that can be pathogenic (Olafsen, 2001). The microorganisms are usually associated with other members of the plankton, such as brine shrimp, which are commonly administered as natural food (Burford et al., 2004). The use of artificial feed requires special care since their nutritional composition to the frequency of feeding. The exhibition area of the pellet feed can serve as a substrate for microbial growth (Ju, 2007). In this study we observed that C. auratus is a species tolerant to adverse water conditions, perhaps with increased stock densities, the recirculation system could play some positive role on the zootechnical performance of cultured animals. The brine shrimp biomass proved to be more effective, but its more expensive, difficult to storage and can serve as a substrate for potential pathogens, what can limit its use. The static water system with the administration of natural food presented the highest number of bacteria in comparison to the recirculation system with provision of commercial food, what lead us to believe that brine shrimp is a possible vehicle for microorganisms contamination.
Acknowledgements
The Brazilian Office for Higher Education Improvement (CAPES), the Brazilian Research Council (CNPq) and the Foundation for Scientific Support and Development of Ceará (FUNCAP), for the financial assistance provided over the research. Ind. e Com. de Alimentos Desidratados Alcon Ltda (Camboriú-SC) and GUABI NUTRIÇÃO ANIMAL by providing inputs from the partnership forged with our research institution.
References
Bandyopadhyay, P., Swain, S.K. and Mishra, S. 2005. Growth and dietary utilisation in goldfish (Carassius auratus Linn.) fed diets formulated with various local agro-produces. Biores. Technol., 96: 731-740. [ Links ]
Barros, H.P. and Valenti, W.C. 2003. Ingestion rates of Artemia nauplii for different larval stages of Macrobrachium rosenbergii. Aquaculture, 217: 223-233. [ Links ]
Burford, M. A., Sellars, M.J., Arnold, S.J., Keys, S.J., Crocos, P.J. and Preston, N.P. 2004. Contribution of the natural biota associated with substrates to the nutritional requirements of the post-larval shrimp, Penaeus esculentus (Haswell), in high-density rearing systems. Aquac. Res., 35: 508-515. [ Links ]
Buschmann, A.H., Lopez, D.A. and Medina, A. 1996. A review of the environmental effects and alternative production strategies of a marine aquaculture in Chile. Aquac. Eng., 15: 397-421. [ Links ]
Chapman, F.A. 2000. Ornamental fish culture freshwater In: R.R. Stickney (Ed.). Encyclopedia of aquaculture. John Wiley and Sons Inc. New York. 1063 pp. [ Links ]
Colt, J., Lamoureux, J., Patterson, R. and Rogers, G. 2006. Reporting standards for biofilter performance studies. Aquac. Eng., 34: 377-388. [ Links ]
Downes, M.P. and Ito, K. 2001. Compendium of methods for the microbiological examination of foods. 4th ed. American Public Health Association. Washington DC. USA. 600 pp. [ Links ]
Gentry, A., Clutton-Brock, J. and Groves, C.P. 2004. The naming of wild animal species and their domestic derivates. J. Arch. Sci., 31: 645-651. [ Links ]
Gouveia, L., Rema, P., Pereira, O. and Empis, J. 2003. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquac. Nutr., 9: 123-129. [ Links ]
Greiner, A.D. and Timmons, M.B. 1998. Evaluation of the nitrification rates of microbead and trickling filters in an intensive recirculating tilapia production facility. Aquac. Eng., 18: 189-200. [ Links ]
Gutierrez-Wing, M.T. and Malone, R.F. 2006. Biological filters in aquaculture: Trends and research directions for freshwater and marine applications. Aquac. Eng., 34: 163-171. [ Links ]
Halachmi, I. 2006. Systems engineering for ornamental fish production in a recirculating aquaculture system. Aquaculture, 259: 300-314. [ Links ]
Hand, S.C. and Podrabsky, J.E. 2000. Bioenergetics os diapauses and quescence in aquatic animals. Thermochim. Acta, 349: 31-42. [ Links ]
Hu, M., Wang, Y., Luo, Z., Zhao, M., Xiong, B., Qian, X. and Zhao, Y. 2008. Evaluation of rendered animal protein ingredients for replacement of fish meal in practical diets for gibel carp, Carassius auratus gibelio (Bloch). Aquac. Res., 39: 1475-1482. [ Links ]
Johnson, S.C., Treasurer, J.W., Bravo, S., Nagasawa, K. and Kabata, Z. 2004. A review of the impact of parasitic copepods on marine aquaculture. Zool. Stud., 43: 229-243. [ Links ]
Joshi, V.P. and Vartak, V.R 1999. A simple method for Artemia (brine shrimp) cyst production. Fish. Chimes, 19: 26-31. [ Links ]
Ju, Z.Y., Forster, I., Conquest, L., Dominy, W., Kuo, W.C. and Horgen, F.D. 2007. Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquacul. Res., 39: 118-134. [ Links ]
Kestemont, P. 1995. Influence of feed supply, temperature and body size on the growth of goldfish Carassius auratus larvae. Aquaculture, 136: 341-349. [ Links ]
Lewbart, G.A. 2001. Bacteria and ornamental fish. Seminars in Avian and Exotic Pet Medicine, 10: 48-56. [ Links ]
Lim, L,C., Dhert, P. and Sorgeloos, P. 2003. Recent developments in the application of live feeds in the freshwater ornamental fish culture. Aquaculture, 227: 319-331. [ Links ]
Linnaeus, C. 1758. Tomus, I. Systema naturae per regna tri naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. reformata. Salvius (Ed.) Holmiae. [ Links ]
Lochmann, R.T. and Phillips, H. 1994. Dietary protein requirement of juvenile golden shiners (Notemigonus crysoleucas) and goldfish (Carassius auratus) in aquaria. Aquaculture, 128: 277-285. [ Links ]
Lovell, R.T. 2000. Nutrition of ornamental fish. In: Bonagura, J. (Ed.). Kirk's Current Veterinary Therapy XIII. Small Animal Practice. W.B. Saunder. Philadelphia, PA. pp. 1191-1196. [ Links ]
Marengoni, N.G., Passos Neto, O.P., Silva Neto, A.A, Silva, A.I.M and Ogawa, M. 2010. Desempenho e proporção sexual de tilápia vermelha sob à inclusão de probiótico em água mesohalina. Arch. Zootec., 59: 403-414. [ Links ]
Naylor, R.L., Goldburg, R.J., Primavera, J.H., Kautsky, N., Beveridge, M.C.M., Clay, J., Folke, C., Lubchenco, J., Mooney, H. and Troell, M. 2000. Effect of aquaculture on world fish supplies. Nature, 405: 1017-1024. [ Links ]
Olafsen, J.A. 2001. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture, 200: 223-247. [ Links ]
Paripatananont, T., Tangtrongpairoj, J., Sailasuta, A. and Chansue, N. 1999. Effect of astaxanthin on the pigmentation of goldfish Carassius auratus. J. World Aquacult. Soc., 30: 454-460. [ Links ]
Sales, J. and Janssens, G.P.J. 2003. Nutrient requirements of ornamental fish. Aquatic Living Res., 16: 533-540. [ Links ]
Schalch, S.H.C. and Moraes, F.R. 2005. Seasonal distribution of gill parasites in different species of fish in fish-pay the City of Guariba-SP, Brazil. J. Vet. Parasitol., 14: 141-146. [ Links ]
Sorgeloos, P., Coutteau, P., Dhert, P., Merchie, G. and Lavens, P. 1998. Use of brine shrimp, Artemia ssp., in larval crustacean nutrition: a review. Rev. Fish. Sci., 6: 55-68. [ Links ]
Sorgeloos, P., Dhert, P. and Candreva, P. 2001. Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture, 200: 147-159. [ Links ]
Tlusty, M. 2001. The benefits and risks of aquacultural production for the aquarium trade. Aquaculture, 205: 203-219. [ Links ]
Whittington, R.J. and Chong, R. 2007. Global trade in ornamental fish from an Australian perspective: The case for revised import risk analysis and management strategies. Prev. Vet. Med., 81: 92-116. [ Links ]
Xue, M. and Cui, Y.B. 2001. Effect of several feeding stimulants on diet preference by juvenile gibel carp (Carassius auratus gibelio), fed diets with or without partial replacement of fish meal by meat and bone meal. Aquaculture, 198: 281-292. [ Links ]
Yanar, M., Erçen, Z., Hunt, A.O. and Büyükçapar, H.M. 2008. The use of alfalfa, Medicago sativa, as a natural carotenoid source in diets of goldfish, Carassius auratus. Aquaculture, 284: 196-200. [ Links ]
Zhou, L., Wang, Y. and Gui, J.F. 2001. Molecular analysis of silver crucian carp (Carassius auratus gibelio Bloch) clones by SCAR markers. Aquaculture, 201: 219-228. [ Links ]
Recibido: 17-9-10
Aceptado: 8-2-11.