Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Archivos de Zootecnia
versión On-line ISSN 1885-4494versión impresa ISSN 0004-0592
Arch. zootec. vol.62 no.239 Córdoba sep. 2013
https://dx.doi.org/10.4321/S0004-05922013000300008
Effect of slaughter handling conditions and animal temperament on bovine meat quality markers
Efecto del manejo y del temperamento animal sobre indicadores de calidad de la carne bovina
Pighin, D.G.1, 3, 4*; Davies, P.2; Grigioni, G.1, 3, 4; Pazos, A.A.1, 4; Ceconi, I.2; Mendez, D.2; Buffarini, M.2; Sancho, A.1, 4 and Gonzalez, C.B.1, 3, 5
1Instituto Tecnología de Alimentos, CIA - CNIA - INTA. Morón. Buenos Aires. Argentina.
2EEA INTA General Villegas. Buenos Aires. Argentina. *dpighin@cnia.inta.gov.ar
3Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Buenos Aires. Argentina.
4Facultad de Agronomía y Ciencias Agroalimentarias. Universidad de Morón. Morón. Buenos Aires. Argentina.
5Escuela de Ciencia y Tecnología. Universidad Nacional de San Martín. San Martín. Buenos Aires. Argentina.
The present research was funded by National Institute of Agropecuary Technology (INTA).
SUMMARY
The aim of this research was to study the effect of different handling conditions on physiological stress indicators and meat quality of beef cattle by studying animals with different temperaments. Forty animals classified by their temperament (calm and disturbed) were used. They were fed on pastures and finished with a mixed diet of corn grain and pasture. Biochemical indicators of animal stress were measured at slaughter (packed cell volume -PCV-, proteins, glucose, creatinine, alkaline phosphatase -APactivity, cortisol, insulin, glycogen). Also, ultimate pH and instrumental colour were chosen as meat quality markers. Animal temperament showed a significantly increase (p<0.05) on PCV levels and a significantly (p<0.05) decrease on muscle glycogen. Besides, levels of plasma glucose and total proteins showed significant (p<0.05) differences associated to management applied. Meat quality markers (pH and colour) did not show significant differences according to handling conditions or temperament. Mean cortisol levels at the exsanguination time were significantly higher (p<0.05) than the values obtained one week prior to slaughter, which suggests an important effect of stress associated to slaughter procedures. It would be interesting to focus attention on the assessment of acute stress at abattoir, in order to improve handling protocols, and therefore to assure meat quality in Argentinean beef production systems.
Key words: Animal stress. Biochemical parameters.
RESUMEN
El objetivo del presente trabajo fue estudiar el efecto de diferentes condiciones de manejo sobre indicadores fisiológicos de estrés y de calidad de carne en bovinos con temperamentos contrastantes. Se utilizaron cuarenta animales clasificados como calmos y excitables. Los mismos fueron alimentados inicialmente a base de pasturas y terminados con una dieta mixta de grano de maíz y pasturas. Se dosaron indicadores bioquímicos de estrés (hematocrito, proteínas plasmáticas, glucosa, creatinina, actividad fosfatasa alcalina, cortisol, insulina, contenido muscular de glucógeno). Como indicadores de calidad de carne se midieron el pH de 24 h y color instrumental. El temperamento animal demostró un incremento significativo (p<0,05) en los niveles de hematocrito y con una disminución significativa (p<0,05) del glucógeno muscular. Los niveles de glucemia y de proteínas totales mostraron modificaciones significativas (p<0,05) asociadas con el manejo. El incremento (p<0,05) en los niveles plasmáticos de cortisol durante la faena, independientemente del tratamiento o el temperamento animal, sugiere un importante efecto estresor por parte del proceso de faena. Sería interesante centrar la atención en el estudio del estrés agudo, a fin de mejorar protocolos de manejo animal, y consecuentemente, optimizar la calidad de la carne asociada en los sistemas de producción de Argentina.
Palabras clave: Estrés animal. Parámetros bioquímicos.
Introduction
Argentinean beef production system is mainly focused in the central area of the country, containing a great number of abattoirs. Animals are usually submitted to short and medium time journeys. Under these conditions, transport stress itself would not represent the only major impact on meat quality. Thus, other management situations, including time of restriction prior to transport, water access and resting time at abattoir´s installations, would emerge as significant aspects, especially when animals have different temperaments (Ferguson and Warner, 2008).
European countries use to slaughter animals the same day of arrival, others -including Argentina- typically slaughter animals after a several hours-lairage. Nowadays, the effect of time of lairage on meat quality and animal welfare is controversial. As pointed out by Del Campo et al. (2010), arguments range between positive effects and negative ones. Positive effects are mainly associated to muscle glycogen replenishment during lairage, while negative effects are related to an inability of recovery associated to a novel environment.
Animal physiology stress can be changed by several factors (genotype, animal temperament, pre mortem handling conditions, etc.). These factors are the responsible for the activation of a neuroendocrinal response to restore the physiological status quo and maintaining life. Whether this physiological response fails to overcome, psychological distress appears leading to several negative effects that impair animal health (Moberg, 2000).
Animal stress can be assessed by the observation of animals' behavior or by means of biochemical parameters. Nowadays, several biochemical tests (cortisol, catecholamines, packed cell volume (PCV), glucose, insulin, creatinine, alkaline phosphatase (AP) activity and muscle glycogen) are used to study the effect of a stressor based on the physiology of the animal (Shaw and Tume, 1992; Cooper et al., 1995; Tadich et al., 2005; Amtmann et al., 2006; López-Olvera et al., 2006). Some authors reported that increased levels of cortisol in blood is one of the most important stress indicators, while others differ and suggest that monitoring cortisol could lead to incorrect interpretation of the results (Grandin, 1997; Moberg, 2000). Muscle glycogen displays a key role in the anaerobic glycolysis of the muscle, adequate levels of glycogen produces an optimal final pH in meat (Purchas et al., 1999).
It is important to remark that the factors that affect animal welfare are able to change the quality of meat. Furthermore, if we pay attention to these factors in order to meet international demands on meat quality, we will avoid the appearance of future technical barriers to global beef trade. Several authors have reported (Gallo et al., 2000; Dunshea et al., 2005) that aspects involved in animal handling, transport conditions (overcrowding, length of journey), adverse climatic conditions, fasting and water deprivation were capable of inducing animal stress, affecting in consequence the final quality of meat.
Animal temperament is defined as the expression of the fearfulness of an animal in response to a challenging situation. This is another key factor to take into account when stress affects meat quality. Voisinet et al. (1997) demonstrated that cattle with an excitable temperament had an extensive response against stressing challenges. The authors concluded that excitable animals could have higher active mechanism response to stressing conditions. Moreover, temperament dissimilarities could display differences in meat tenderness as a result of the modification of post mortem proteolytic mechanisms (King et al., 2006b).
Falkenberg et al. (2005) and King et al. (2006b) reported that meat obtained from excitable cattle had higher incidence of dark cutting and higher Warner-Bratzler shear force values, when it was compared to calm animals.
Nowadays, parameters commonly measured to evaluate meat quality are ultimate pH (Watanabe et al., 1996) and colour, being colour one of the most important components used by consumers to assess acceptability and contribute to their first purchasing decision.
The aim of the research was to study the effect of different handling conditions on biochemical stress indicators and on meat quality parameters in beef cattle by studying animals with contrasting temperaments.
Materials and Methods
ANIMALS
Seventy Angus steers bred in the field of the experimental station of INTA General Villegas (Buenos Aires, Argentina) from July 2006 were initially evaluated. Their initial average weight was 161 ± 28 kg. Animals were fed with pasture; triticale and lucerne (forage levels: 2.5 y 3 % of live weight, respectively) during the first 15 months. The daily average weight gain (ADG) was 0.550 kg/day. In November 2007, their diet was changed for a mixture of corn and pasture (60 % and 40 %, respectively), which was administered at 1.8 % of live weight. During this period, ADG was 0.474 kg/day.
Steers with an average final weight of 439 ± 27 kg were transported to a commercial abattoir licensed for exporting meat, where they were humanely sacrificed. Procedures stated by SENASA (Handbook of Procedures for Animal Welfare of the National Service of Animal Health) were applied for animal handling and experimental studies.
TREATMENTS
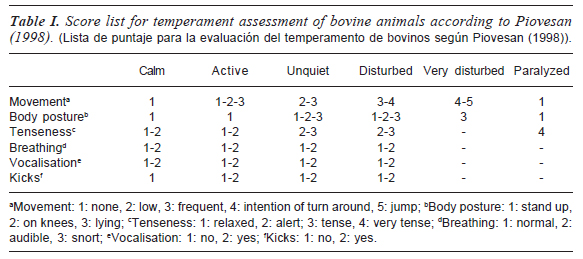
Seventy animals were classified according to their temperament by means of a behavioral score method (Piovesan, 1998; Barbosa Silveira et al., 2008). This method was based on the animals' reaction when they were restrained in a reduced space and let to obtain a composite score. It is important to remark that this methodology of temperament classification has been recently significantly correlated to other methods like Flight Distance and Flight Speed (Barbosa Silveira et al., 2008; 2010), both of them also used on different production animals and systems.
In order to classify the animals into six temperament degrees, each animal was placed in a scale and was observed to obtain several scores for different aspects of the behavior (movement, body posture, tenseness, breathing, vocalization, and kicks) (table I). Observation was carried out during 4 seconds by two judges positioned outside of the scale, without applying any external stimulus.
After the scoring, forty animals that according to table I belonged to calm (C) and disturbed (D) categories were selected. Those animals were randomly divided into two groups containing the same number of C and D animals. Each group was submitted to two different management ways: management with pre-slaughter rest (MR), and management without pre-slaughter rest (MWR). MR group was deprived from water for 12 h, transported to the slaughterhouse at 170 km distance and lairaged for 20 h with free access to water. MWR group was enclosed in a pen during 20 h prior to transport (first 12 h without water and then with free access to water). Then, they were transported under the same conditions -truck, stock density, driving conditions- than MR group and, finally, they were lairaged for 6 h without water access.
SAMPLE COLLECTION
Animals were stunned using a captive bolt pistol and exsanguinated. Blood samples were collected one week prior to stress treatments and during exsanguination in tubes and immediately placed on ice. Plasma (with EDTA) and serum (without anticoagulant) were obtained by centrifugation at 2000 rpm for 10 min and kept at -20 ± 1oC until analysis. An aliquot of anticoagulated blood (EDTA) was used to determine PCV immediately after the slaughter procedure. Samples destined to basal biochemical analysis -with the exception of cortisol- could not be used due to chilling malfunction during storage.
Muscle samples for glycogen determination were obtained post mortem within two hours post mortem. Small pieces of Supraspinatus muscle were cut, immediately frozen in liquid nitrogen, and kept under -80 oC until analysis.
Left carcasses were chilled in the abattoir at 4 ± 1 oC for 24 h. After measuring ultimate pH, three-ribs blocks (11th to 13th rib) were removed, vacuum packaged and maintained under -20 ± 1 oC until analysis.
BLOOD CONSTITUENT'S LEVEL
PCV was measured by the microhematocrit technique (Cavour centrifuge, Argentina) and expressed as percentage (%, v/v). Plasma protein concentration was determined according to Bradford method (Bradford, 1976), using bovine serum albumin (SIGMA, USA) as standard. Plasma glucose level was assayed by GOD/POD Trinder Colour test without deproteinization (GT Lab, Argentina), and expressed as mM. Plasma creatinine concentration was measured using a kinetic method kit (GT Lab, Argentina) and was expressed as mg/L. Serum Alkaline Phosphatase (AP) activity was measured using a kinetic method test (GT Lab, Argentina) and expressed as IU/L. Cortisol plasma level was measured by ECL test (Modular E 170, Roche), while insulin was measured by ELISA test (DSL, USA). Intra and inter-assay coefficients of variation were 3 % and 5 % for cortisol, and 3 % and 6 % for insulin.
MUSCLE GLYCOGEN CONTENT
The level of available muscle glycogen was measured by acid hydrolysis method (Passonneau and Lauderdale, 1974). Briefly, about 500 mg of muscle samples were homogenized (Ultraturrax, Fisher Scientific) for 30 s in 5 mL 2 N HCl, and then, submitted to hydrolysis at 100 ± 1 oC for 2 h. Glucose released was measured in the neutralized homogenates (2 N NaOH) with the GOD/POD Trinder Color test (GT Lab, Argentina). Available glycogen content was expressed as mmol of glucose per gram of wet tissue. The measurement included free glucose and glucose-6-phosphate.
The selection of Supraspinatus muscle for glycogen assay was based in two facts. First, it is an oxidative muscle mainly involved in constant motion and position. It has been stated that this type of muscle suffers from physical stress during transport to the slaughterhouse and/or when resting conditions are reduced (Lacourt and Tarrant, 1985). The second approach is that this slow-twitch muscle has a greater number of β-adrenergic receptors compared to the glycolytic one, which in turn provides an increased sensitivity to catecholamines (Jensen et al., 1995).
MEAT PHAND COLOR
Muscle ultimate pH (24 h post mortem) was measured in the Longissimus dorsi (LD) muscle (12th rib level) using a portable pHmeter (Thermo Orion model 420, USA) with a standardized combination electrode.
After thawing for 24 h at 4 ± 1 oC, colour measurements were carried out in a BYK Gardner Spectro-guide 45/0 gloss Spectrophotometer (USA), using a D65 standard illuminant and 10o observer geometry, following the recommendations of AMSA (2012). Determinations were carried out in 2.5 cm thick steaks obtained from the 13th rib. CIE Lab system provides the values of three color components: L* (black-white component, luminosity); and the chromaticness coordinates, a* (+ red to - green component) and b* (+ yellow to - blue component). The instrument was calibrated against white and black plates (plate numbers: 6811 and 6843 BYK Gardner). Each sample was allowed to bloom for 45 min, at the end the four scans from each steak were averaged for statistical analysis.
STATISTICAL ANALYSIS
A completely randomized block design was considered (RCBD) with the primary objective of estimating and comparing treatment's means. The treatment fixed effect was represented by the management conditions (MR and MWR). The random block effect was represented by the temperament classification (C and D). Samples taken one week prior to slaughter were considered as the baseline covariate in the statistical analysis of cortisol levels. PROC MIXED and LSMEANS statements were performed with SAS® (v.8.02) for Mixed Models statistical package (Littell et al., 2006).
Results and Discussion
BLOOD CONSTITUENTS
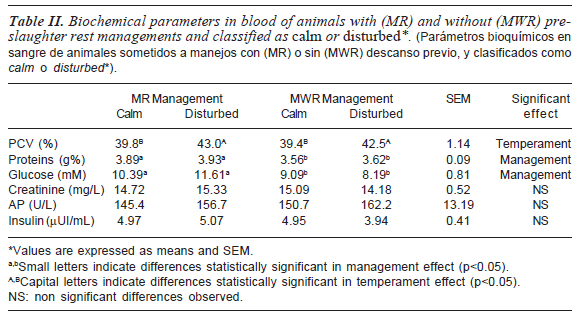
Table II shows the effect of temperament and handling applied on biochemical parameters at the exsanguination time. As previously stated, no basal data could be recorded -with the exception of cortisoldue to a freezing malfunction of samples. Nevertheless, the metabolic status of the animals was observed by means of data collected at slaughter time.
PCV values showed a significant effect associated to animal temperament (p<0.05). Disturbed animals submitted either to MR or MWR displayed significantly increased PCV values when compared to calm countermates. Regarding this issue, it is well known that PCV provides information related to the blood concentration, and it is usually used as an indicator of dehydration. Increasing levels of PCV can be attributed to a dehydration mechanism or it can be a consequence of the release of red blood cells by the spleen, which is usually related to an increase of catecholamines induced by stress.
Total protein concentration is considered a useful measure to find out which mentioned mechanism was mainly operating. In the current study, total protein concentration was significantly associated to management conditions (p<0.05), rather than to temperament. Animals submitted to MWR management showed lower levels of total proteins when compared to MR. Several authors have reported (Tarrant et al., 1992; Earley and O'Riordan, 2006) that changes in protein concentration may be due to several factors, including time of journey and fasting among others. Recently, a decrease in plasma total proteins in young beef bulls has been associated to transport stress (Buckham Sporer et al., 2008). Even though in the present assay all the animals were submitted to similar transport conditions, it is possible that a protein metabolism alteration could have taken place during this period. Probably, animals submitted to MR handling partially restored its protein profile during resting at abattoir.
The fact that PCV and total protein concentration displayed different behavior on statistical significance suggests that separated mechanisms are involved. This reinforces the idea that the increased PCV values observed in disturbed animals would be more associated to the splenic contraction induced by the increased circulating catecholamines (Warriss et al., 1995; Gupta et al., 2007) than to dehydration mechanisms.
Considering glucose basal levels widely known for ruminants, all tested animals showed hyperglycemia at exsanguination time. The glucose increment could be related to the acute stress induced by the knocking. Hyperglycemia found in animals subjected to MWR was lower (p<0.05) than the corresponding value in MR-treated animals (groups C and D), indicating that the level of glucose in the blood at the time of the bleeding depends on the pre mortem stress intensity. This increment of circulating glucose can be the consequence of the rapid breakdown of hepatic glycogen as a result of increased levels of adrenaline and noradrenaline (Warriss, 2000).
Creatinine levels and AP activity did not show significant differences among animals. However, blood AP activity of temperamental animals was higher than in calm ones. The lack of changes in creatinine levels would indicate that the treatments applied had no direct impact on physical stress (e.g. increment of non-enzymatic breakdown of muscle creatine) or on kidney activity (e.g. reduction of glomerular filtration rate). Therefore, it would be possible to consider that animal handling applied in the present study mainly affected the psyche of animals rather than its physique. Supporting these results, Ndlovu et al. (2008) also found no significant differences in serum creatinine, when different breeds or transport treatments were used. Taking into account the current discussion, it is also possible that creatinine is not only non-specific but also non sensitive enough for handling conditions applied in the present research.
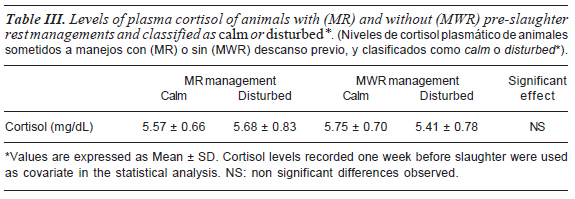
Regarding hormone concentrations, non significant differences were found in cortisol (table III) or insulin concentrations (table II) among treatments. Mean cortisol levels at the exsanguination time (7.04 mg/dL) were significantly higher (p<0.05) than the values obtained one week prior to slaughter (3.15 mg/dL).
Considering the cortisol rank proposed by Grandin (1997), mean cortisol levels obtained at the slaughter moment could be compared to those values detected in animals held in head-gates. This comparison supports the idea that the high levels of cortisol observed at slaughter could be the result of stress induced by pre-mortem handling. Cortisol levels found in the present research are in accordance with previous published data (Tadich et al., 2005), which showed non different response against diverse stress conditions at slaughter time.
Instead, King et al. (2006b) showed that serum cortisol concentrations differed among animals belonging to different temperament categories, serum cortisol concentration decreased when excitability decreased. Cortisol levels recorded in the current research also suggest that animals belonging to contrasting temperaments displayed similar stress response against slaughter procedures. These results suggests that under present conditions, acute stress of slaughter procedures would be more relevant to the animal physiology than managements conditions applied, even when animals from contrasting temperaments are being considered.
Concerning insulin concentrations, McVeigh et al. (1982), in accordance with the present results, did not found changes in serum insulin in animals submitted to more stressing conditions. Taking into account that these hormones are responsible for major metabolic effects and are known to be counter-regulated, the lack of differences in the other blood and muscular constituents assayed seems to be reasonable.
MUSCLE GLYCOGEN CONTENT
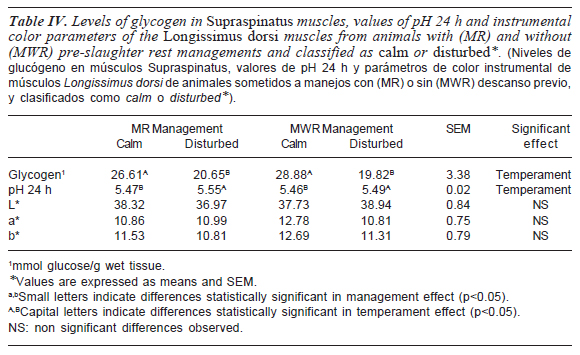
Table IV shows the effect of the different managements conditions on the glycogen content of Supraspinatus muscle within 2 h post mortem. Muscle glycogen content was significantly affected by the animals' temperament (p<0.05). On this regard, it is known that muscle glycogen concentration depends on several factors including breed (King et al., 2006a), nutritional status (Andersen et al., 2005), temperament (King et al., 2006b), pre-slaughter stress and physiological responsiveness to stress (O'Neill et al., 2006), replenish capacity, and resting level (Wiklund et al., 1996). Under particular conditions, some of these factors are able to change glycogen levels in living muscles, and therefore, post mortem pH decrease and/or ultimate pH, generating defects on the quality of meat (Apple et al., 1995).
In the present study, animals belonging to a stronger temperament due to classification showed decreased in muscle glycogen content (p<0.05), independently of the management applied. Despite none dark-cutting conditions were observed, this particular point should be taken as an early indicator of increased stress susceptibility. Generally, mean glycogen levels found were lower than expected according to previous published research (Tarrant, 1989; Pighin et al., 2011). This could be possibly related to the metabolic characteristics of the Supraespinatus muscle, classified as a slow-twitch oxidative muscle.
MEAT PHAND COLOR
The ultimate pH values of LD muscles are shown in table IV. The final pH of calm animals was significantly (p<0.05) lower than pH of disturbed steers. However, ultimate pH of all assayed animals was between normal values, independently of the management treatment applied or animal temperament.
Ultimate pH is commonly measured to evaluate ante mortem animal handling and to predict meat quality; previous reports (Fernandez et al., 1996; Lensink et al., 2001) have shown that some ante mortem events -e.g. feed withdrawal before transport, and transport duration- might not be always reflected in the ultimate pH of beef.
Results in this research showed that the normal ultimate pH and the low glycogen level found in the muscle of disturbed animals would agree with the medium stress concept, proposed by Immonen and Poulanne (2000), which involves a combination of an adequate final pH and low residual carbohydrate levels. Thus, despite meat quality was not affected in the present study, the potential negative effect of low residual glycogen should be analyzed in the future.
LD instrumental colour values (table IV) were not affected by handling conditions or by animal temperament. This finding was not unexpected since pH 24 h values obtained in this study did not differ among treatments, suggesting that processes of meat quality development by means of pH-dependent mechanisms were not compromised.
Results obtained are in agreement with King et al. (2006b), who also reported a lack of modifications in quality grade factors or colour of carcasses of feed-lot steers with different temperament. On the other hand, obtained data is in contrast to results presented by Voisinet et al. (1997). These authors have reported a relationship between excitable temperaments and borderline dark-cutting. In the present study, none of the animals displayed dark-cutting condition. However, considering that the present work did not include ageing evaluation, any late effect of handling treatments upon meat quality could not be discarded.
In the last years, emerging data proposes that non-pH mediated effects on meat quality could occur as a result of stress treatment (Ferguson and Warner, 2008). Future research is warranted to study the possible impact of stress and/or temperament on the quality of meat along ageing process.
Conclusion
Stress response of beef cattle to different management conditions simulating expected circumstances of the central area of Argentina did not show significant differences on meat quality neither when it was associated to animals temperament.
Temperament was associated to early biochemical changes: increased PCV and decreased muscle glycogen, which supported the idea that special care should be taken for managing. Plasma glucose and protein concentration were associated to management conditions, suggesting the possibility of a favorable effect of resting time before slaughter. The increment of plasma cortisol levels at slaughter, independently of management or temperament characteristics, suggested an important effect of stress associated to slaughter procedures, a clearly key issue to improve.
Further research is warranted in order to evaluate acute handling stress and handling protocols in order to improve welfare perspective in the Argentinean beef production systems.
Acknowledgements
Authors express their gratitude to MSc. Natalia Aguilar for her contribution on the field related to temperament characterization, and to Mrs. Cecilia Barreto, Mr. Luis Sanow and Mrs. Monica Pecile for their technical assistance in the lab techniques. Authors also thank to Dra. Valeria Messina, for reviewing the manuscript and collaborating with the English edition. Cooperation and assistance of staff at abattoir Trenel (La Pampa, Argentina) is also gratefully acknowledged.
References
1. Andersen, H.A.; Oksbjerg, N.; Young, J.F. and Therkildsen, M. 2005. Feeding and meat quality -a future approach. Meat Sci, 70: 543-554. [ Links ]
2. Amtmann, V.A.; Gallo, C.; Van Schaik, G. y Tadich, N. 2006. Relaciones entre el manejo antemortem, variables sanguíneas indicadoras de estrés y pH de la canal en novillos. Arch Med Vet, 38: 1-10. [ Links ]
3. AMSA. 2012. Meat color measurement guidelines. American Meat Science Association. http://www.meatscience.org (01/08/2012). [ Links ]
4. Apple, J.K.; Dikeman, M.E.; Minton, J.E.; McMurphy, R.M.; Fedde, M.R.; Leith, D.E. and Unruh, J.A. 1995. Effects of restraint and isolation stress and epidural blockade on endocrine and blood metabolite status, muscle glycogen metabolism, and incidence of dark-cutting Longissimus muscle of sheep. J Anim Sci, 73: 2295-2307. [ Links ]
5. Barbosa Silveira, I.D.; Fischer, V. e Soares, G.J.D. 2008. Efeito do grupo etnológico no temperamento de novillos mantidos em condições extensivas. Arch Zootec, 57: 123-129. [ Links ]
6. Barbosa Silveira, I.D.; Fischer, V. e Wiegand, M.M. 2008. Temperamento em bovinos de corte: Métodos de medida em diferentes sistemas productivos. Arch Zootec, 57: 321-332. [ Links ]
7. Barbosa Silveira, I.D.; Fischer, V. e Mendonça, G. 2010. Efeito do genótipo e da idade de ovinos na reatividade medida em pista de venda. Rev Bras Zootecn, 39: 2304-2309. [ Links ]
8. Bradford, M.M. 1976. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72: 248-254. [ Links ]
9. Buckham Sporer, K.R.; Weber, P.S.D.; Burton, J.L.; Earley, B. and Crowe, M.A. 2008. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J Anim Sci, 86: 1325-1334. [ Links ]
10. Cooper, C.A.; Evans, C.O.; Cook, S. and Rawlings, N.C. 1995. Cortisol, progesterone and βendorphin response to stress in calves. Can J Anim Sci, 95: 197-201. [ Links ]
11. Del Campo, M.; Brito, G.; Soares de Lima, J.; Hernandez, P. and Montossi, F. 2010. Finishing diet, temperament and lairage time effects on carcass and meat quality traits in steers. Meat Sci, 86: 908-914. [ Links ]
12. Dunshea, F.R.; D'Souza, D.N.; Pethick, D.W.; Harper, G.S. and Warner, R.D. 2005. Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Sci, 71: 8-38. [ Links ]
13. Earley, B. and O'Riordan, E.G. 2006. Effects on transporting bulls at different space allowances on physiological, haematological and immunological responses to a 12-h journey by road. Ir J Agr Food Res, 45: 39-50. [ Links ]
14. Falkenberg, S.M.; Miller, R.K.; Holloway, J.W.; Rouquette, F.M. Jr.; Randel, R.D. and Carstens, G.E. 2005. Exit velocity effects on growth, carcass characteristics, and tenderness in half-blood Bonsmara steers. In: Proceedings 51st International Congress of Meat Science and Technology, 7-12 August. Baltimore, MD. p. 29. [ Links ]
15. Ferguson, D.M. and Warner, R.D. 2008. Have we underestimated the impact of pre-slaughter stress on meat quality in ruminants? Meat Sci, 80: 12-19. [ Links ]
16. Fernandez, X.; Monin, G.; Culioli, J.; Legrand, I. and Quilichini, Y. 1996. Effect of duration of feed withdrawal and transportation time on muscle characteristics and quality in Freisan-Holstein calves. J Anim Sci, 74: 1576-1583. [ Links ]
17. Gallo, C.; Perez, S.; Sanhueza, C. y Gasic, J. 2000. Efectos del tiempo de transporte de novillos previo al faenamiento sobre el comportamiento, las pérdidas de peso y algunas características de la canal. Arch Med Vet, 32: 157-170. [ Links ]
18. Grandin, T. 1997. Assessment of stress during handling and transport. J Anim Sci, 75: 249-257. [ Links ]
19. Gupta, S.; Earley, B. and Crowe, M.A. 2007. Effect of 12-hour road transportation on physiological, immunological and haematological parameters in bulls housed at different space allowances. Vet J, 173: 605-616. [ Links ]
20. Jensen, J.; Brors, O. and Dahl, H.A. 1995. Different beta-adrenergic receptor density in different rat skeletal muscle fibre types. Pharmacol Toxicol, 76: 380-385. [ Links ]
21. Immonen, K. and Poulanne, E. 2000. Variation of residual glycogen-glucose concentration at ultimate pH values below 5.75. Meat Sci, 55: 279-283. [ Links ]
22. King, D.A.; Morgan, W.W.; Miller, R.K.; Sanders, J.O.; Lunt, D.K.; Taylor, J.F.; Gill, C.A. and Savell, J.W. 2006a. Carcass merit between and among family groups of Bos indicus crossbred steers and heifers. Meat Sci, 72: 496-502. [ Links ]
23. King, D.A.; Schuehle Pfeiffer, C.E.; Randel, R.D.; Welsh, T.H.; Oliphint, R.A.; Baird, B.E.; Curley, K.O. Jr.; Vann, R.C.; Hale, D.S. and Savell, J.W. 2006b. Influence of animal temperament and stress responsiveness on the carcass quality and beef tenderness of feedlot cattle. Meat Sci, 74: 546-556. [ Links ]
24. Lacourt, A. and Tarrant, P.V. 1985. Glycogen depletion pattern in myofibres of cattle during stress. Meat Sci, 15: 85-100. [ Links ]
25. Lensink, B.J.; Fernandez, X.; Gozzi, G.; Florand, L. and Veissier, I. 2001. The influence of farmer behaviour on calves reactions to transport and quality of veal meat. J Anim Sci, 79: 642-652. [ Links ]
26. Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D. and Schabenberger, O. 2006. SAS® for Mixed Models. 2nd ed. 2006. SAS Institute Inc. Cary, NC. USA. [ Links ]
27. López-Olvera, J.R.; Marco, I.; Montane, J. and Lavín, S. 2006. Transport stress in Southern chamois (Rupicapra pyrenaica) and its modulation by acepromazine. Vet J, 172: 347-355. [ Links ]
28. McVeigh, J.M.; Tarrant, P.V. and Harrington, M.G. 1982. Behavioral stress and skeletal glycogen metabolism in young bulls. J Anim Sci, 54: 790-795. [ Links ]
29. Moberg, G.P. 2000. Biological response to stress: implications for animal welfare. In: G.P. Moberg & J.A. Mench (Eds.). The biology of animal stress. Basic principles and implications for animal welfare. CABI Publishing. Oxon. UK. pp. 1-22. [ Links ]
30. Ndlovu, T.; Chimonyo, M.; Okoh, A.I. and Muchenje, V. 2008. A comparison of stress hormone concentrations at slaughter in Nguni, Bonsmara and Angus steers. Afr J Agric Res, 3: 96-100. [ Links ]
31. O'Neill, H.A.; Webb, E.C.; Frylinck, L. and Strydom, P.E. 2006. The stress responsiveness of three different beef breed types and the effect on ultimate pH and meat colour. In: Proccedings 52nd International Congress of Meat Science and Technology. 13-18 August 2006. Dublin. Ireland. pp. 181-182. [ Links ]
32. Passonneau, J.V. and Lauderdale, V.R. 1974. A comparison of three methods of glycogen measurement in tissues. Anal Biochem, 60: 405-412. [ Links ]
33. Pighin, D.G.; Warner, R.D.; Jacob, R.; Beatty, D.; Naththarampatha, A. and Ferguson, D. 2011. The impact of long term grain feeding on glycolytic metabolism of cattle. In: Proccedings 57th International Congress of Meat Science and Technology. 7-12 August 2011. Ghent. Belgium. [ Links ]
34. Piovesan, U. 1998. Análise de fatores genéticos e ambientais na reatividade de quatro raças de bovinos de corte ao manejo. Dissertação (Mestrado em Zootecnia). Faculdade de Ciências Agrárias e Veterinárias. Universidade Estadual Paulista. Jaboticabal-SP. 51 f. [ Links ]
35. Purchas, R.W.; Yan, X. and Hartley, D.G. 1999. The influence of a period of ageing on the relationship between ultimate pH and shear values of beef m. Longissimus thoracis. Meat Sci, 51: 135-141. [ Links ]
36. Shaw, F.D. and Tume, R.K. 1992. The Assessment of pre-slaughter and slaughter treatments livestock by measurement of plasma constituents. A review of recent work. Meat Sci, 32: 311-329. [ Links ]
37. Tadich, N.; Gallo, C.; Bustamante, H.; Schwerter, M. and Van Schaik, G. 2005. Effects of transport and lairage time on some blood constituents of Friesian-cross steers in Chile. Livest Prod Sci, 9: 223-233. [ Links ]
38. Tarrant, P.V. 1989. Animal behaviour and environment in the dark-cutting condition. In: Dark-cutting in cattle and sheep. Eds S.U. Fabiansson, W.R. Shorthose, R.D. Warner. Australian Meat and Livestock Research and Development Corporation. Sydney. pp. 8-18. [ Links ]
39. Tarrant, P.V.; Kenny, F.J.; Harrington, D. and Murphy, M. 1992. Long distance transportation of steers to slaughter: Effect of stocking density on physiology, behavior, and carcass quality. Livest Prod Sci, 30: 223-238. [ Links ]
40. Voisinet, B.D.; Grandin, T.; O'Connor, S.F.; Tatum, J.D. and Deesing, M.J. 1997. Bos indicus-cross feedlot cattle with excitable temperaments have tougher meat and a higher incidence of borderline dark cutters. Meat Sci, 46: 367-377. [ Links ]
41. Watanabe, A.; Daly, C.C. and Devine, C.E. 1996. The effects of the ultimate pH of meat on tenderness changes during ageing. Meat Sci, 42: 67-78. [ Links ]
42. Warriss, P.D.; Brown, S.N.; Knowles, T.G.; Kestin, S.C.; Edwards, J.E.; Dolan, S.K. and Phillips, A.J. 1995. Effects on cattle of transport by road for up to 15 hours. Vet Rec, 136: 319-323. [ Links ]
43. Warriss, P.D. 2000. Meat science: an introductory text. In: P.D. Warris, Ed. Animal Welfare. CABI Publishing. London. UK. pp. 209-228. [ Links ]
44. Wiklund, E.; Andersson, A.; Malmfors, G. and Lundström, K. 1996. Muscle glycogen levels and blood metabolites in reindeer (Rangifer tarandus tarandus L.) after transport and lairage. Meat Sci, 42: 133-144. [ Links ]
Recibido: 29-12-11.
Aceptado: 13-2-13.