Introduction
Hyperuricemia plays a major role in the development and progression of chronic kidney disease (CKD).1 Recent studies suggest mechanisms of damage of hyperuricemia other than the traditional precipitation of urate into the tubules. In animal models, hyperuricemia induces the development of a glomerular arteriolopathy that impairs renal autoregulation and causes glomerular hypertension, leading eventually to glomerulosclerosis and interstitial fibrosis. Both factors are well-known related to progression of kidney disease.2 Epidemiological studies have recently shown the association between serum uric acid levels and increased risk of developing CKD.3,4 Even after adjusting for the classical comorbidities that favour kidney damage (proteinuria, hypertension or dislypemia), the predictive value of hyperuricemia in the progression of CKD remains as an independent factor.5-7 This association between hyperuricemia and kidney disease progression has been showed in some types of nephropathies including Ig A nephropathy,8,9 autosomal-dominant polycystic kidney disease,10 diabetes nephropathy11 and renal transplantation.12,13
The long-term outcome of individuals with reduce functioning renal mass has been a subject widely studied,14,15 and several observational studies have shown conflicting results.16-21 All of these studies vary with respect to type of solitary functioning kidney studied and inclusion criteria. It is therefore difficult to draw conclusions. In addition, the number of longitudinal prospective studies is limited because of the decades required for follow up.
Hyperuricemia may be a modifiable risk factor for the progression to end stage renal disease (ESRD) in CKD patients. We performed this observational longitudinal study in a cohort of patients with reduced functional renal mass to evaluate whether hyperuricemia is associated with faster progression of chronic kidney disease. Besides, as secondary objective, we analyzed the evolution of chronic kidney disease in the different etiologies of reducing kidney mass.
Materials and methods
Three hundred and thirty-seven prevalent patients with reduced kidney mass were included in this single centre retrospective study. Patients seen on clinic between January 2012 and December 2012 were included. Inclusion criteria were: 1) patients older than 18 years, 2) reduced functioning renal mass due to nephrectomy, unilateral renal agenesis or unilateral kidney atrophy and 3) outpatient follow-up greater than one year. All patients were attended at least once a year during the follow-up time. Demographics variables as age, sex, cardiovascular factors: hypertension, diabetes mellitus (DM), dyslipemia, coronariopathy, peripheral vascular disease and cerebrovascular disease and concomitant medications were recorded. Hypertension was defined as being treated with antihypertensive medication or seated BP ≥ 140/90 mmHg in at least 50% of blood pressure measurements at the beginning of the study. Pulse pressure (systolic arterial minus diastolic arterial pressure) was calculated. Antihypertensive medication was recorded yearly. Dislipemia was defined as being treated with statins or total-cholesterol > 200 mg/dl or LDL-cholesterol > 130 mg/dl or triglycerides > 150 mg/dl. Hyperuricemia was defined as uric acid level above 7 mg/dl. Blood sample was drawn to determine routine serum chemistry values and 24-h-urine collection was obtained to measure proteinuria yearly. Variables were monitored at least yearly during the follow-up time. Routine clinical and biochemical variables were measured on autoanalyzers using standardized methods. Highly sensitive creatinine in plasma (CRP) was measured using a latex-based turbidimetric immunoassay on a Hitachi analyzer (Sigma Chemical Co, St. Louis, Missouri, USA). Daily urinary albumin excretion was measured using an immunonephelometric method. The 4-variable Modification of Diet in Renal Disease (MDRD) Study equation (IDMS-traceable) was used to measure eGFR.
The primary endpoint was the annual fall of eGFR by MDRD-4. First, according to the decrease of GFR, we divided the patients into two groups and analyze the differences: group A patients with fall of GFR during follow-up and group B patients without fall of GFR during follow-up. Second, in function to the median of fall of GFR, the group A was divided into two subgroups: A1 patients with fall of GFR lower than the median and group A2 patients with fall of GFR greater than the median. Besides, we analyzed factors related to renal disease progression in patients with nephrectomy in comparison to those with renal agenesis or atrophy.
Statistical analysis
All statistical analyses were performed using IBM SPSS, version 21.0 (IBM Corp, Armonk, New York, USA) for Windows XP. Values are expressed as mean ± SD or median (interquartile range). Categorical data were compared using the chi-square test and quantitative variables using the t test. Kaplan-Meier curves and log-rank test were used to analyze renal survival in function to hyperuricemia and renal mass reduction. Cox proportional hazard models were used to evaluate the risk of CKD progression and results were adjusted for several covariates. Age, gender and baseline renal function were introduced in the model as potential confounders covariates. Univariate Cox regression was used to determine which covariates should be introduced in the multivariable model. Statistical significance was defined as a 2-tailed p value of less than 0.05.
Results
Three hundred and twenty four patients (175 males, 149 females) were included consecutively. The median follow-up time was 60 (36-98) months. The aetiology of the reduced functioning renal mass was: nephrectomy (n = 181, 55.86%), unilateral renal agenesis (n = 118, 36.42%) and unilateral kidney atrophy (n = 27, 8.3%).
Five patients progressed to dialysis and 13 patients doubled serum creatinine. One hundred and seventy out of 324 patients suffered a fall of eGFR (group A) and 154 patients did not modify their GFR along follow-up time, or even increased it (group B). Differences in analytical and cardiovascular factors between both groups are showed in Table 1. Male gender (p = 0.001), albuminuria > 100 mg/day (p = 0.02) and higher pulse pressure (p = 0.025) accelerated renal disease progression. Patients with nephrectomy had lower kidney disease progression than patients with unilateral renal agenesis or atrophy (p = 0.022).
Table 1 Baseline characteristics and cardiovascular factors between patients with renal disease progression (group A) vs patients without renal disease progression (group B).

No differences in LDL and HDL-cholesterol and statins use were found between groups.
The median GFR decline among patients of group A was −1.6 ml/min/1.73 m2/year (−3.0-0.7 ml/min/1.73 m2/year). Those patients with GFR fall lower than −1.6 ml/min/1.73 m2 were nominated group A1 and the rest of patients group A2. Differences in analytical and cardiovascular factors between both subgroups are showed in Table 2. Again, pulse pressure increased renal disease progression (p = 0.035). Hyperuricemia was more frequent among patients with higher renal disease progression (33% vs 49%, p = 0.04) and the use of diuretics was not greater in these patients (39% vs 43%, p = 0.277).
Table 2 Baseline characteristics and cardiovascular factors in patients with renal disease progression group A1: lower than 1.6 ml/min/1.73 m2 and group A2: higher than median.

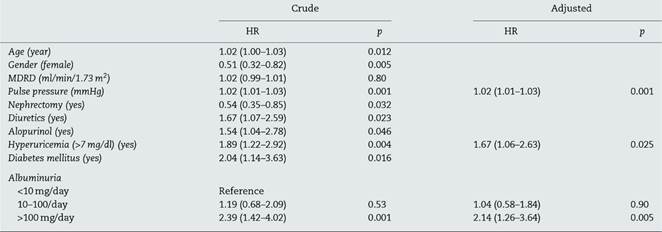
Kidney disease progression (defined as a fall greater than 1.6 ml/min/1.73 m2) was higher in patients with hyperuricemia (>7 mg/dl), log rank: 8.851, p = 0.003 as shown in Fig. 1. The multivariate Cox regression analysis was done including all the parameters that were statistically significant in the univariate Cox analysis (diuretics use, diabetes mellitus, aetiology of renal mass reduction, albuminuria and pulse pressure), and some parameters considered confounding variables as age, gender and previous renal function (Table 3). This model showed an association between showed hyperuricemia and kidney disease progression (HR 1.67 (1.06-2.63), p = 0.023. Albuminuria and higher pulse pressure were also independently associated with kidney disease progression: HR: 2.14 (1.26-3.64), p = 0.005 and 1.02 (1.01-1.03), p = 0.001, respectively (Table 3).

Fig. 1 Relationship between hyperuricemia (>7 mg/dl of uric acid level) and renal disease progression (Cox regression model).
Discussion
This longitudinal study describes for the first time that hyperuricemia could play an important role in the progression of renal disease in patients with reduced functioning kidney mass. It is an extensive group of patients with renal mass loss in which the renal involvement is mostly generated by hyperfiltration, without glomerular, toxic or systemic damage. That allows to study risk factors in these kinds of patients and compare the differences in the CKD progression between patients with nephrectomy and patients with renal atrophy or agenesis.
Solitary functioning kidney can be congenital or acquired and is a frequent renal pathology.22 Both types of renal mass reduction can be associated with ESRD and early differentiation between patients with and without risk of ESRD progression is challenging. The clinical importance of a reduced nephron number has been described in the hyperfiltration hypothesis by Brenner more than three decades ago. In experimental studies, Brenner demonstrated that a reduced functional nephron number results in a compensatory glomerular hypertension and enlargement of remnant nephrons causing glomerular hyperfiltration. Individuals with a solitary functioning kidney have a reduction in renal mass that therefore can be at risk of hyperfiltration injury and progression to ESRD.23,24
In this longitudinal long-term study, we observe that patients with reduced functioning kidney mass have a slow progression to end-stage renal disease. During a median of 60 months, only 5 patients progressed to dialysis. In addition, our analysis shows that hyperuricemia together with hypertension and albuminuria are associated with kidney disease progression in these reduced functioning kidney mass patients.
Important differences in renal outcome may exist between congenital and acquired solitary functioning kidneys. Congenital solitary kidney still has the potential to form new nephrons whereas acquired solitary kidney has probably ceased nephrogenesis capacity when nephrectomy is performed. This finding may imply a higher susceptibility for pronounced glomerular hyperfiltration in acquired solitary functioning kidney patients and consequently higher rates of progression. Abou Jaoudé et al. reported a lower GFR in children with an acquired solitary functioning kidney than children with a congenital solitary functioning kidney (mean GFR: 95 versus 107 ml/min/1.73 m2, respectively). Nevertheless, this data does not agree with our results, probably due to the lack of information about the time elapsed since the loss of renal mass.
The prognosis of a solitary functioning kidney in childhood was analyzed in The Kidney of Monofunctional Origin (KIMONO) study. This longitudinal follow-up study from The Netherlands includes over 400-children with both types of solitary functioning kidney: congenital and acquired. Subjects were routinely screened for markers of renal injury, defined as hypertension, albuminuria and/or decline of glomerular filtration rate. The analyses showed that nearly one in three patients with solitary functioning renal mass has signs of renal injury at a mean age of 10 years. Based on findings on the KIMONO study a differentiation between patients with and without high risk for CKD should be made at diagnosis. Besides of control of blood pressure and anti-proteinuric medication, our results showed that it will probably be necessary to control uric acid levels and avoid hyperuricemia to delay CKD progression. Recently, hyperuricemia has been described as an important role in the progression of chronic kidney disease. Experimental studies in the remnant kidney rat model showed that mild hyperuricemia accelerated preexisting renal disease, by promoting glomerulosclerosis, interstitial fibrosis and arteriolosclerosis.25 Allopurinol prevented severe histological changes in this model. The potential mechanisms by which hyperuricemia leads to progression of renal disease are the induction of endothelial dysfunction,26 inflammation and oxidative stress.27-30 These effects occur mainly because uric acid stimulates the renin-angiotensin system and inhibits vascular nitric oxide synthesis.31 Clinical effect has been proven in two randomized trials in patients with chronic kidney disease, which have shown that allopurinol use for 1-2 years slowed progression compared with the control group.1,11,32 In observational studies, hyperuricemia has been implicated in the progression of renal disease of patients with IgA nephropathy,8,9 polycystic kidney disease,10 diabetic nephropathy11 and renal transplant.12,13 Some studies have also proven the role of uric acid in the endothelial dysfunction generation.26 These studies cannot clarify whether the reduction of uric acid or the blocking of the xanthine oxidase enzyme is the mechanism implicated in the progression of CKD. Therefore, more experimental studies are needed to clarify whether uric acid is an objective to reduce the progression of chronic kidney disease in different etiologies, including reduced renal mass.
Our study has some limitations. First, it is a retrospective study. Secondly, patients are included from the first visit in the nephrology department, which do not imply the same time of nephrectomy or lifelong in the case of patients with renal agenesis or renal atrophy. This is likely to involve a bias in the selection of patients that can modify the results. And finally, we have only one initial determination of uric acid, though the uric acid level progression reminds unknown.
In conclusion, our study suggests that hyperuricemia may play a role in the progression of chronic kidney disease in patients with reduced functioning renal mass and that it could be a therapeutic target. More experimental studies would be needed to support this observation.