Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.18 no.2 Madrid mar./abr. 2003
Revisión
Pharmacological Nutrition in Inflammatory Bowel Diseases
F. G. Campos, D. L. Waitzberg, M. G. Teixeira, D. R. Mucerino, D. R. Kiss and A. Habr-Gama
Department of Gastroenterology. Colorectal Surgery Unit. Hospital das Clinicas. University of São Paulo Medical School. Sao Paulo, Brasil.
| Abstract Inflammatory Bowel Diseases - ulcerative colitis and Crohns disease- are chronic gastrointestinal inflammatory diseases of unknown etiology. Decreased oral intake, malabsorption, accelerated nutrient losses, increased requirements, and drug-nutrient interactions cause nutritional and functional deficiencies that require proper correction by nutritional therapy. The goals of the different forms of nutritional therapy are to correct nutritional disturbances and to modulate inflammatory response, thus influencing disease activity. Nutritional intervention may improve outcome in certain individuals; however, because of the costs and complications of such therapy, careful selection is warranted. Total parenteral nutrition has been used to correct and prevent nutritional disturbances and to promote bowel rest during active disease, mainly in cases of digestive fistulae with a high output. Its use should be reserved for patients who cannot tolerate enteral nutrition. Enteral nutrition is effective in inducing clinical remission of disease in adults and promoting growth in children. Recent research has focused on the use of specific nutrients as primary treatment agents. Although some reports have indicated that glutamine, short-chain fatty acids, antioxidants and immunonutrition with omega-3 fatty acids are an important therapeutic alternative in the management of inflammatory bowel diseases, the beneficial reported effects have yet to be translated into the clinical practice. The real efficacy of these nutrients still need further evaluation through prospective and randomized trials. (Nutr Hosp 2003, 18:57-64) Keywords: Immunonutrients. Inflammatory bowel disease. Nutritional therapy.
| NUTRICIÓN FARMACOLÓGICA Resumen Las enfermedades inflamatorias del intestino -colitis ulcerosa y enfermedad de Crohn- son enfermedades crónicas de causa desconocida. La disminución de la ingesta, la malabsorción, la pérdida acelerada de nutrientes, el aumento de los requerimientos y las interacciones entre medicamentos y nutrientes determinan carencias nutricionales y funcionales que obligan a una corrección mediante terapia nutricional. Los objetivos de los distintos tipos de terapia nutricional comprenden la corrección de las alteraciones nutricionales y la modulación de la respuesta inflamatoria para modificar la actividad mórbida. La alimentación puede mejorar algunos aspectos pero, debido al coste y a las complicaciones de este tratamiento, exige una cuidadosa selección. Se ha empleado la nutrición parenteral total para corregir y prevenir las alteraciones nutricionales y fomentar el reposo del intestino durante la actividad, sobre todo en los casos de fístulas digestivas con un elevado drenaje. Su uso debe reservarse a los pacientes que no toleren la nutrición enteral. La nutrición enteral induce una remisión clínica eficaz entre los adultos y promueve el crecimiento infantil. La investigación reciente se ha centrado en el uso de nutrientes específicos como medios esenciales de tratamiento. Si bien en algunos informes se ha señalado que la glutamina, los ácidos grasos de cadena corta, los antioxidantes y la inmunonutrición con ácidos grasos omega-3 suponen una alternativa terapéutica importante para el tratamiento de las enfermedades inflamatorias del intestino, los efectos beneficiosos descritos todavía no se han reflejado en la práctica clínica. La eficacia real de estos nutrientes obliga a una evaluación más cuidadosa a través de ensayos prospectivos y aleatorizados. (Nutr Hosp 2003, 18:57-64) Palabras clave: Enfermedad inflamatoria del intestino. Inmunonutrientes. Terapia nutricional. |
Correspondencia: Rua Padre João Manuel, 222.
Sãao Paulo. Brazil (CEP: 01420-002).
Correo electrónico: fgcampos@osite.com.br
Tel.: 55-11-30640654 - Fax: +55-11-3081.1443
Recibido: 16-VII-2002.
Aceptado: 17-VIII-2002.
Introduction
Inflammatory Bowel Diseases (IBD) are often associated with significant nutritional disturbances, such as protein-calorie malnutrition, vitamin and trace element deficits. Such problems are aggravated by complications occurring during the evolution of the disease, like bowel obstruction, intestinal resections and disease activity. Thus, appropriate nutritional management of IBD patients is an essential part of their treatment.
The prevalence of IBD associated-malnutrition is high, ranging from 23% in outpatients to 85% in inpatients admitted for clinical exacerbation1, 2. Malnutrition is influenced by disease activity, length and site of inflammation. Nutritional deficits are more common in small bowel CD than when inflammation is confined to the colon3.
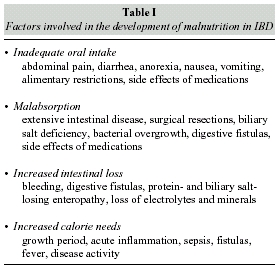
Adequate nutritional care for IBD patients requires identification and correction of malnutritionassociated factors, represented by either local and systemic alterations or drug side effects (table I). The reduction of dietary intake is the main cause of malnutrition.
Nutritional deficits occur in variable incidences, such as anemia (54 to 80%), hypoalbuminemia (25 to 80%), metals (iron, copper), trace elements (selenium, magnesium, zinc), vitamins (A, B, D, E, K) and reduction of enzymatic (superoxyde dismutase, catalase, gluthatione peroxydase) and non-enzymatic antioxidant activity (vitamins C, E, ß-carotene, gluthatione, taurine).
Nutritional deficits are associated with adverse clinical outcome, affecting cellular and humoral immunity, linear body growth and sexual maturation in children, fistula and wound healing, nitrogen balance and bone decalcification. Moreover, reduced blood loss tolerance, greater postoperative morbidity rates and slower functional recovery can occur4, 5.
In active Crohns Disease (CD) patients, weight loss results from anorexia, malabsorption and intestinal losses, rather than hypermetabolism. As reintroduction of normal dietary intake can reverse metabolic changes, the importance of NT in this population is well recognized6. Patients with CD develop malnutrition slowly, with frequent severe deficiencies. On the other hand, patients with ulcerative colitis (UC) usually preserve nutritional status, but can develop severe deficiencies very fast due to disease activity. For these reasons, the most appropriate management of IBD requires attention to nutritional aspects from the initial diagnosis.
When analyzing nutrition and IBD, three major aspects must be considered: the influence of nutritional components in its pathogenesis, the impact of IBD on nutritional status and the potential role of nutritional therapy (NT). Although important advances have been recently achieved in the understanding of IBD pathogenesis, there is still no consensus regarding the indications and standards of NT for these patients.
This article reviews the role and efficacy of specific nutrients used as pharmacological nutritional agents in IBD.
Principles of Nutritional Therapy in IBD
It does not exist a single uniformly effective dietary protocol for patients with IBD7. The majority of outpatients can usually adopt liberal dietary intake of calories and proteins, provided that some restrictions might be necessary based on individual intolerance4.
Oral, enteral and parenteral nutritional therapy might be necessary during the different phases of IBD. When calorie and protein intakes do not match the needs for maintaining body mass in adults and adequate growth in children, some more effective nutritional intervention must be tried, such as the administration of liquid oral supplements. When the risk of malnutrition persists, the benefits and risks of enteral or parenteral nutrition must be considered.
The main goals of NT are the maintenance and/or recovery of nutritional status, remission of disease activity, reduction of surgical indications and postoperative complications8. Generally, the enteral route is preferred since it is associated with fewer complications and lower costs, thus saving the parenteral route for patients with contra-indications or intolerance for enteral feeding.
Specialized NT is indicated in acute, severe and recurrent exacerbation, preoperative preparation of malnourished patients, digestive tract fistulas, short bowel syndrome (anatomic or functional) and growth retard. Usually, many of those indications are based on clinical expertise rather than on clinical trials results9.
Reviews of uncontrolled studies in malnourished patients reveal that preoperative total parenteral nutrition (TPN) reduces complications and the extent of intestinal resection, even though increasing hospital admission lengths7. The benefits of bowel rest, TPN and elemental diets in patients with refractory CD have been known for more than 25 years, including bet- ter nutritional status, symptomatic relief and even temporarily complete clinical remission10. The use of NT for controlling symptoms and signs of a disease is called primary nutritional therapy.
Both CD and UC exhibit different responses to enteral and parenteral nutritional support. During the last years, excellent literature reviews11-13 and metaanalyses14, 15 concerning primary NT of IBD have been published.
Total Parenteral Nutrition (TPN) and Enteral Nutrition (EN)
TPN aims are preoperative bowel rest, fulfilling postoperative nutritional requirements and correcting malnutrition. It can al so be used as primary therapy for active and severe IBD2. In other cases, TPN can be used as a complement to poorly tolerated or quantitatively insufficient oral or enteral nutrition to maintain the patients nutritional status or correct malnutrition16.
The use of primary TPN and bowel rest for acute active IBD is still controversial, since total bowel rest is not essential for disease remission17, although it reduces mucosal inflammation and disease activity. During acute toxic colitis, TPN maintains protein reserves, provides calories and reduces surgical complications18.
The presented data indicates that TPN should be offered to IBD patients in need for nutritional therapy who do not tolerate the enteral route, especially during acute and severe disease courses. The available experience supports the use of TPN for at least 5 days to correct severe preoperative malnutrition in elective surgical situations or at least 1-3 days in cases of intense disease activity19, 20.
TPN is also effective as primary treatment in refractory CD, even though a higher late recurrence rate is observed when compared to surgical treatment21. TPN has favorable clinical response in cases of ileitis22, 23 and it is essential in malnourished patients and when one previews a fasting period greater than 5 days. Numerous reports about the effects of TPN in intestinal fistulas reveal initial closure rates of 44%2. Indeed, low output cutaneous fistulas may have some benefit from TPN, reducing morbidity and improving local conditions for surgical treatment7. On the other hand, primary TPN is not effective in the treatment of complex CD fistulas and UC. Long-term home TPN plays a major role in improving the quality of life of patients with CD and severe short bowel syndrome.
The use of TPN adds significant costs and length to hospital admissions, especially when septic, metabolic or venous access complications occur24. For these reasons, it has gradually lost space in face of the benefits of nutrient provision directly to the mucosa25.
In patients with UC, TPN is not considered an effective primary treatment. Prospective and retrospective studies showed remission rates lower than 40% (initial) and 10 to 30% (late)2.
In general, EN should be preferred to TPN in NT. As primary therapy to active CD, data suggest that EN has equal efficacy compared to TPN, but it is less effective compared to corticosteroids. EN plays an important role in selected cases, unresponsive to usual treatment, or in children and teenagers.
Although there is no difference in remission rates between elemental, oligomeric and polymeric diets, it is known that polymeric diets are more efficient concerning the improvement of nutritional state14, 15. Furthermore, the similar early remission rates of enteral diets and TPN in CD suggest that bowel rest provided by TPN does not influence the efficacy of treatment26 and the benefits of these regimens were due to nutritional improvement17.
Despite the benefit of early remission induction on active CD, further data are necessary for better evaluation of enteral diet therapy on CD complicated by fistula and stenosis.
Enteral nutrition is not efficient as primary therapy for clinical remission of ulcerative colitis patients27.
Pharmacological Nutrition in IBD
The recovery from trauma, infection and inflammation depends on the bodys ability to prioritize physiological events, activating several metabolic paths. Some pathologic processes are associated with an increase of inflammatory mediators that sometimes aggravates the primary damage. Immune suppressive therapies in IBD are based on its physiopathogenesis, aiming to decrease inflammation by controlling mediators synthesis and target organ response to these mediators. Anabolic factors, growth and tissue regeneration control mechanisms have drawn less attention, whether during the acute phase or during remission periods.
In IBD, some factors may lead to intestinal epithelial cells damage, and some nutrients are important to maintain intestinal structure and function (table II). Treatment of gastrointestinal diseases with specific nutrients is a new therapeutic modality based on their pharmacological properties. This concept is called pharmacological nutrition.
In IBD, a rational plan must include nutrients to provide calories, to induce low antigenic stimuli, to regulate inflammatory and immunologic responses and to stimulate mucosal trophism.28, 29. The present status regarding the utilization of specific nutrients in the treatment of IBD is discussed below.
Glutamine
Glutamine (GLN) is the most common aminoacid in mammals blood. It is synthesized in almost all tissues, especially in striated muscle. Once released by the muscle, GLN acts as a nitrogen carrier and beco- mes part of a great number of proteins. Furthermore, it is an important energy source for fast proliferation cells such as fibroblasts, lymphocytes, neoplastic cells and intestinal epithelium cells30.
It is considered the main oxidative fuel of epithelial cells, especially jejunal enterocytes31. In vitro studies showed that colonocytes also preferentially metabolize GLN instead of glucose32.
Although GLN is a non-essential aminoacid, experimental and clinical data suggest that it may become conditionally essential in catabolic states31, 33. Conventional TPN solutions and enteral nutrition do not provide adequate amount of GLN to a catabolic patient. Thus, GLN supplementation may improve structural integrity, function and intestinal recovery in catabolic conditions associated to radiation therapy, chemotherapy and sepsis34. Moreover, it prevents intestinal atrophy during TPN and after intestinal resections, maintains mucosal barrier and has antioxidant properties in inflammation35.
In experimental models of colitis, it was demonstrated that GLN enteral supplementation might reduce endotoxemia, improve mucosal barrier function mucosa36 and even exacerbate colitis37.
In the few clinical trials available, GLN prevented intestinal permeability increase in active IBD patients that received postoperative TPN38.
On the other hand, there was no effect on intestinal permeability or disease activity in CD patients who received prolonged GLN supplementation39. Good results were reported with topical use of L- GLN in patients with pouchitis who received this treatment for 21 days40.
Although limited, the available data suggest that GLN is an important trophic nutrient to the intestinal mucosa. However, there is still no clear evidence of its therapeutic role on IBD7, 35.
Short Chain Fatty Acids
Short chain fatty acids (SCFA) are organic fatty acids constituted by 1 to 6 carbons derived from the bacterial degradation of dietary carbohydrate41, 42. More than 90% of the SCFA found in humans are acetate, propionate and butyrate.
Nowadays it is a consensus that SCFA have an important role in normal colon physiology, once they are the main energy source to the colonocyte. They also stimulate cellular proliferation, visceral blood flow and enhance sodium and water absorption from the intestinal lumen. Butyrate is the main oxidative fuel of colonocytes, representing 70% of the total energy consumption43, 44.
The data concerning SCFA role on physiopathology and treatment of ulcerative colitis (UC) are contradictory. Roediger45 first noted that UC patients had lower butyrate oxidation levels, suggesting that luminal factors were responsible for the oxidation impairment. These findings indicated that a butyrate functional deficiency could play a role in UC pathogenesis, leading to inflammation and ulceration.
For this reason, topical provision of SCFA was tested with positive results, suggesting its use as an alternative therapy in distal refractory colitis to decrease disease activity43, 46. However, these findings were later questioned in newer studies in which alterations in butyrate concentrations werent observed in mild or severe UC patients44, 47.
The association between SCFA and pouchitis after restorative proctocolectomy (RPC) is also a matter of discussion in literature. The construction of an ileal pouch has become the treatment of choice in UC and familial adenomatous poliposis patients. Pouchitis is defined as a non-specific inflammation of the ileal reservoir occurring after RPC. Its etiology is controversial- infectious causes, immunologic causes, fecal stasis, UC recurrence, Crohns Disease, and mucosal ischemia have been considered48, 49. The occurrence of fecal stasis leading to changes in luminal environment (bacterial, bile acids action and SCFA alterations) has been investigated41, 50, 51.
Sandborn et al.51 reported that SCFA concentrations in the ileal pouch fluids are similar in patients with or without pouchitis. However, other studies have found lower fecal SCFA levels in patients with pouchitis50, 52, similarly to active UC43. Sagar et al.52 noted that metronidazole treatment reestablishes SCFA concentration in pouch fluids.
It has been suggested that pouchitis and UC have a common etiology, UC recurrence being proposed as a cause of pouchitis53. Active UC is characterized by low SCFA fecal concentration. Clausen et al.50 believe that the inflammation in pouchitis and UC is triggered by a decrease in SCFA bacterial synthesis and mucosal metabolism. According to them, a vicious cycle occurs, in which pouchitis-induced diarrhea decreases the fermentation, dilutes the available carbohydrates and reduces SCFA synthesis. This chain of events would lead to impaired absorption of water and sodium and diarrhea.
Available data concerning butyrate levels in UC patients are still controversial, and more research is necessary to determine the SCFA role in the etiology and treatment of UC. The cause-effect association between SCFA low levels, impaired absorption and number of bowel movements is still speculative, and the association between SCFA and pouchitis deserves new controlled trials for better comprehension.
Omega-3 Fatty Acids (Fish oil)
Lipids are usually present in enteral and parenteral formulas as fat emulsions (FE). Several fatty acids (FA) are associated to the etiology and treatment of colorectal diseases, and may have influence on the immunological system function and in colonocyte metabolism54, 55.
Although the inflammatory cascade is considered to be a protective event, it may become a threat when it is too exacerbated or when immunological disorders are present. So, inflammatory response modulation by pharmacologically balanced FE is a therapeutic modality with great perspectives. In this scenario, FE supplemented with omega-3 FA has received great attention to study its potential role on nutritional therapy in several conditions56, 57.
Eisosapentaenoic acid (EPA) and docosahexacnoic acid (DHA) are polyunsaturated FA extracted from fish oil, also known as omega-3 (n-3 or w-3), once its first double carbon ligation is located in the third carbon from the methyl radical. As arachidonic acid (n- 6), EPA and DHA are also incorporated to the cellular membrane phospholipid layer.
The exogenous provision of fish oil-derived FA promotes a fast enhancement of n-3 FA plasmatic concentrations, mainly if it is given parenterally58. Thus, an extracellular enzymatic competition is established between n-3 FA and arachidonic acid (AA), decreasing pro- inflammatory mediators synthesized from AA and increasing the concentration of less potent inflammatory mediators synthesized from n-3 FA59.
In other words, there is a decrease in series 2 prostanoids and series 4 leukotrienes, and an increase in series 3 and 5, respectively (fig. 1). These mediators have different structural and biological activities than their arachidonic acid derived-analogs, with considerably lower potency regarding inflammatory response, aggregation and vasoconstriction properties56, 60. Moreover, there is also a decrease in 1-beta- interleukin synthesis and in tumor necrosis factor-alpha secretion61.
The first evidence of the n-3 FA ingestion relevance came from epidemiological studies that showed low incidence of inflammatory diseases in Eskimos. More recently, a study conducted in Japan showed that DC incidence is strongly linked to dietary n-6 FA/n-3 FA relation, suggesting that larger intake of n- 6 FA may contribute to the development of this disease62.
Treatment with oral or parenteral n-3 FA in IBD patients has had favorable results in clinical trials and experimental studies, decreasing symptoms, corticosteroid needs, and promoting colonic histological and endoscopic improvement63-66. These effects are attributed to alteration in the inflammatory mediator profile67.
Initial reports about the use of oral n-3 FA in a small number of patients showed benefits in UC, decreasing disease activity, LT synthesis and corticosteroids needs68-70. Lorenz et al.71 conducted first prospective, controlled, double- blind study, in which 29 CD patients with different clinical activity were treated, and did not observe any alteration on clinical activity indexes.
Some randomized, double-blind trials evaluated the role of fish oil supplementation versus placebo in UC patients. Stenson et al.72 observed weight gain, histological improvement in rectal biopsies, and a 61% decrease in LTB4 levels in rectal dialysate. In another trial, Aslan and Triadafilopoulos64 observed histological improvement, a 56% decrease in disease activity index and no alteration on diarrhea frequency. However, the mucosal LTB4 decrease was 30%, against 26% in a placebo group (no statistical significance). Hawkey et al.65 noted that patients treated with EPA had earlier disease remission, received less steroids, and had an increase in LTB5 and a 50% decrease in LTB4 synthesis. On the other hand, Loeschke et al.73 compared oral n-3 FA (5.1 g/day) to placebo in a small group of UC patients for 2 years, observing no significant difference in clinical activity and histology. Recently, Almallah et al.63 showed that fish oil might improve clinical picture and decrease histological and endoscopic scores when compared to sunflower oil.
Good results have also been reported in CD patients. Belluzzi et al.74 noted remission rates of 59% versus 26% in the placebo group. On the other hand, Lorenz-Meyer et al.75 did not observed any increase on remission periods in 70 CD patients when compared to placebo.
Some factors might have biased final results. Inadequate placebo groups with the use of olive oil, the provision of high fish oil doses (more side effects and less palatability) and different fish oil chemical formulations with different absorption rates76.
To solve these matters, Belluzzi et al.77 developed an enteric-coated free fatty acid mixture, observing better plasmatic absorption and cellular uptake for CD patients. Later on, Belluzzi et al.74 compared the effects of this new preparation to placebo on CD remission status, observing good tolerance and effectiveness in prolonging clinical remission (59 versus 26%).
Some experimental studies tried to detail the alterations on eicosanoid synthesis by the intestinal mucosa after n-3 FA provision. Guarner et al.78 concluded that n-3 FA supplementation attenuates ulcer progression and disease time course in experimental colitis, effects related to lower intraluminal concentrations of TXB2 and LTB4. Shoda et al.79 reported lower LTB4 serum levels and less ulcer formation in rats fed with a 2% peril oil (n-3 FA) enriched-elemental diet. Nieto et al.67 also noted an improvement in histological recovery, lower stenosis index and lower PGE2 and LTB4 levels in the mucosa of TNBS induced-colitis rats fed with n-3 FA when compared to rats fed with n-3 FA plus n-6 FA.
Marotta et al.66 concluded that EPA supplemented diet protects the colonic mucosa from early lipidic and morphologic derangement observed in an acute inflammation model. These effects probably contribute to the maintenance of mucosal barrier integrity.
In a recent study, Geerling et al.80 showed that supplementation with antioxidants improved antioxidant status in patients with CD in remission. In addition, supplementation with n-3 fatty acids plus antioxidants significantly changed the eicosanoid precursor profile. This study indicates that an immunomodulating formula containing n-3 fatty acids and/or antioxidants may have the potential to play a role in the treatment of CD.
Literature data suggest that parenteral n-3 FA leads to more effective and earlier benefits when compared to the enteral route. It is believed that fast changes in plasmatic and membrane FA composition lead to alterations on lipidic mediators synthesis and earlier clinical results.
Ikehata et al.81 offered EPA enriched- LE (0,6 g) for 2 weeks to active CD patients, and noted an increase in LTB5/LTB4 ratio, with no significant alteration in LTB4 synthesis. Grimminger et al.82 reported a quick decrease in acute UC activity, with remarkable control of bleeding and systemic symptoms.
In TNBS induced-colitis studies, Inui et al.83 showed that rats fed for 7 days with alpha-linolenic (n-3 FA) enriched- LE had greater weight gain, increased EPA/AA ratio, suppressed LTB4 colonic synthesis and had milder intestinal lesions when compared to rats fed with soy oil enriched-TPN.
N-3 FA enriched- LE can have modulatory effects in many clinical situations depending on the n-3/n-6 FA ratio29, 60. Supposedly, the decreased immunosuppressive effects and the pharmacological advantages of a balanced LE (n-3/n-6 FA = 1:2 to 1:4) are due to the eicosanoids profile synthesized in response to greater n-3 FA intake57, 84, 85.
In our laboratory at the university, the infusion of a MCT/LCT LE supplemented with n-3 FA in rats with acetic acid induced-colitis was associated with clinical benefits, decreased tissue inflammation, preserved mucosal morphology and lowered eicosanoids synthesis. These effects were more consistent when a 1:3 n-3/n-6 FA ratio was offered86.
The controversial aspects of n-3 FA on nutritional therapy may be a consequence of different study designs and the use of different formulations/doses. Probably, n-3 FA effects are mostly due to its anti-inflammatory properties on active disease rather than to prevention of relapsed disease. The possibility of using this therapeutical approach in all patients with IBD has not been elucidated yet. Despite the good results reported in this review, parenteral utilization of n-3 FA still requires evaluation of other factors such as triglyceride chain dimension, treatment duration, n- 3/n-6 ratio and association to other immune modulator nutrients. Further assessment of efficacy, costs, risks and side effects will bring new lights into the treatment of IBD with immune modulatory therapies87.
Conclusions
Malnutrition in IBD patients is frequent, multifactorial and has many deleterious consequences. Its treatment requires identification of nutritional deficits in order to choose the best nutritional therapy in each situation.
TPN may correct nutritional deficits, maintain nutritional status and serve as primary therapy in patients with active CD. However, because of its high costs and complication rates, EN is the route of choice for nutritional therapy. Several studies attested the efficacy of enteral formulations to control disease activity in CD patients.
Clinical and experimental use of trophic nutrients as glutamine, SCFA, and immune modulator nutrients as n-3 FA brought up new perspectives. Despite positive preliminary results, further prospective and controlled trials are necessary to establish their role in IBD management.
References
1. Gassul MA, Abad A, Cabre E, Gonzalez-Huix F, Gine JJ and Dolz C: Enteral nutrition in inflammatory bowel disease. Gut, 1986, 27:76-80. [ Links ]
2. Han PD, Burke A, Baldassano RN, Rombeau JL and Lichtenstein GR: Nutrition and inflammatory bowel disease. Gastroenterol Clin, 1999, 28:423-436. [ Links ]
3. Lewis JD and Fisher RL: Nutrition support in inflammatory bowel disease. Med Clin N Amer, 1994, 78:1443-1456. [ Links ]
4. Griffiths AM: Inflammatory bowel disease. Nutrition, 1998, 14:788-791. [ Links ]
5. Silva MLT and Waitzberg DL: Terapia nutricional na doença inflamatória intestinal. In: Habr-Gama A (ed.): Doença Inflamatória Intestinal. Atheneu, São Paulo, 1997: 69-79. [ Links ]
6. Schneeweiss B, Lochs H, Zauner C, Fisher M, Wyatt J and Schneider B: Energy and substrate metabolism in patients with active Crohns disease. J Nutr, 1999, 129:844-848. [ Links ]
7. Alpers DH: Use of macro and micronutrients for nutrition support in inflammatory bowel disease. In: Bistrian BR, Walker- Smith JA (eds): Nestle Nutr Workshop Ser Clin Perform Programme, 1999, 2:155-170. [ Links ]
8. Campos ACL and Coelho JCU: Suporte nutricional nas doenças inflamatórias intestinais. Rev Bras Nutr Clin, 1994, 9:55-62. [ Links ]
9. Fell JME, Paintin M, Donnet-Hughes A, MacDonald TT and Walker-Smith JA: Remission induced by a new specific oral polymeric diet in children with Crohns disease. In: Bistrian BR, Walker-Smith JA (eds.): Nestle Nutr Workshop Ser Clin Perform Programme, 1999, 2:187-198. [ Links ]
10. Dieleman LA and Heizer WD: Nutritional issues in inflammatory bowel disease. Gastroenterol Clin N Am, 1998, 27:435- 451. [ Links ]
11. King TS, Woolner JT and Hunter JO: Review article: the dietary management of Crohns disease. Aliment Pharmacol, 1997, 11:17-31. [ Links ]
12. Stenson WK and Alpers DH: Nutritional therapy in Crohns disease: a historical overview. Curr Opin Gastroenterol, 1997, 13:135-139. [ Links ]
13. OSullivan MA and OMorain CA: Nutritional therapy in Crohns disease. Inflammatory Bowel Disease, 1998, 4:45-53. [ Links ]
14. Griffiths AM, Ohlsson A, Sherman PM and Sutherland LR: Meta-analysis of enteral nutrition as primary therapy of active Crohns disease. Gastroenterology, 1995, 108:1056-1067. [ Links ]
15. Fernández-Banares F, Cabre E, Esteve-Comas M and Gassul MA: How effective is enteral nutrition in inducing clinical remission in active Crohns disease? A meta-analysis of the randomized clinical trials. J Parent Enteral Nutr, 1995, 19:356- 364. [ Links ]
16. Messing B: Parenteral nutrition: indications and techniques. Ann Med Interne (Paris), 2000, 151:652-658. [ Links ]
17. Greenberg GR, Fleming CR, Jeejeebhoy KN et al.: Controlled trial of bowel rest and nutritional support in the management of Crohns disease. Gut, 1988, 29:1309-1315. [ Links ]
18. DeWitt RC, Kudsk K: Enteral Nutrition. Gastroenterol Clin N Am, 1998, 27:371-386. [ Links ]
19. ASPEN Board of Directors: Guidelines for use of parenteral and enteral nutrition in adults and pediatric patients. J Parenter Enteral Nutr 17, 1993, (suppl):18S. [ Links ]
20. Christie PM, Graham MB and Hill GL>: Return to normal body composition after ileoanal J-pouch anastomosis for ulcerative colitis. Dis Colon Rectum, 1990, 33:584-586. [ Links ]
21. Scolapio JS: The role of total parenteral nutrition in the management of patients with acute attacks of inflammatory bowel disease. J Clin Gastroenterol, 1999, 29:223-224. [ Links ]
22. Duerksen DR, Nehra V, Bistrian BR and Blackburn GL: Appropriate nutritional support in acute and complicated Crohns disease. Nutrition, 1998, 14:462-465. [ Links ]
23. Seo M, Okada M, Yao T, Furukawa H and Matake H: The role of total parenteral nutrition in the management of patients with acute attacks of inflammatory bowel disease. J Clin Gastroenterol, 1999, 29:270-275. [ Links ]
24. Faintuch J, Waitzberg DL, Bertevello PL, Silva ML, Borges VC, Pereira SS, Gama-Rodrigues JJ and Pinotti HW: Conservative management of septic parenteral nutrition catheters. JPEN, 1995, 19:428-429. [ Links ]
25. Dudrick SJ: Past, present and future of nutritional support. Surg Clin N Am, 1991, 71:439-448. [ Links ]
26. Klein S: Influence of nutrition support on clinical outcome in short bowel syndrome and inflammatory bowel disease. Nutrition, 1995, 2 (suppl):233-237. [ Links ]
27. González-Huix F, Fernández-Banares F and Esteve-Comas M: Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am J Gastroenterol, 1993, 88:227- 232. [ Links ]
28. Hayashi N, Tashiro T, Yamamori H, Takagi K, Suzuki N and Ito I: Effects of intravenous omega-3 fat emulsion on cytokine production and delayed type hypersensitivity in burned rats receiving total parenteral nutrition. JPEN, 1998, 22:363-367. [ Links ]
29. Tashiro T, Yamamori H, Takagi K, Hayashi N, Furukawa K and Nakajima N: N-3 versus n-6 polyunsaturated fatty acids in critical illness. Nutrition, 1998, 14:551-553. [ Links ]
30. Souba WW and Wilmore DW: Gut-liver interaction during accelerated gluconeogenesis. Arch Surg, 1985, 120:66-70. [ Links ]
31. Souba WW: The gut as a nitrogen processing organ in the metabolic response to critical illness. Nutr Sup Serv, 1988, 8:15- 22. [ Links ]
32. Pinkus LM and Windmueller HG: Phosphate-dependent glutaminase of small intestine: localization and role in glutamine metabolism. Arch Biochem Biophys, 1977, 182:506-517. [ Links ]
33. Campos FG, Waitzberg DL, Mucerino DR, Logulo A and Habr-Gama A: Importância da Glutamina em Nutrição Clinica. Rev Gastroenterol Clin, 1996, 10:6-7. [ Links ]
34. Campos FG: Efeitos da glutamina e dieta elementar na enterite actinica aguda - Estudo experimental. Dissertação de Mestrado apresentada à Faculdade de Medicina da Universidade de São Paulo, 1992. [ Links ]
35. Jonas CR and Ziegler TR: Potential role of glutamine administration in inflammatory bowel disease. In: Bistrian BR, Walker-Smith JA (eds.): Nestle Nutr Workshop Ser Clin Perform Programme, 1999, 2:217-235. [ Links ]
36. Fujita T and Sakurai K: Efficacy of glutamine-enriched enteral nutrition in na experimental model of mucosal ulcerative colitis. Br J Surg, 1995, 82:749-751. [ Links ]
37. Shinozaki M, Saito H and Muto T: Excess glutamine exacerbates trinitrobenzenesulfonic acid-induced colitis in rats. Dis Colon Rectum, 1997, 40:S59-S63. [ Links ]
38. Veterans Affairs Total Parenteral Nutrition Cooperative Study Group: Perioperative total parenteral nutrition in surgical patients. N Engl J Med, 1991, 235:525-532. [ Links ]
39. Den Hond E, Hiele M, Peeters M, Ghoos Y and Rutgeerts P: Long-term glutamine supplements have no effect on small intestinal permeability in Crohns disease (abstract). Gastroenterology, 1997, 112:A958. [ Links ]
40. Wischmeyers P, Pemberton JH and Phillips SF: Chronic pouchitis after ileal pouch-anal anastomosis: responses to butyrate and glutamine suppositories in a pilot study. Mayo Clin Proc, 1993, 68:978-981. [ Links ]
41. Ambroze WL, Pemberton JH, Phillips SF, Bell AM and Haddad AC: Fecal short-chain fatty acid concentrations and effect on ileal pouch function. Dis Colon Rectum, 1993, 36:235-239. [ Links ]
42. Clausen MR and Mortensen PB: Kinetic studies on the metabolism of short-chain fatty acids and glucose by isolated rat colonocytes. Gastroenterology, 1994, 106:423-432. [ Links ]
43. Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G and Kasper H: Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology, 1992, 103:51-56. [ Links ]
44. Hove H and Mortensen PB: Influence of intestinal inflammation (IBD) and small and large bowel length on fecal shortchain fatty acids and lactate. Dig Dis Sci, 1995, 40:1372-1380. [ Links ]
45. Roediger WE: The colonic epithelium in ulcerative colitis - an energy deficiency disease? Lancet, 1980, 2:712-715. [ Links ]
46. Breuer RI, Buto SK and Christ ML: Rectal irrigation with short-chain fatty acids for distal ulcerative colitis: Preliminary report. Dig Dis Sci, 1991, 36:185-187. [ Links ]
47. Vernia P, Caprilli R, Latella G, Barbetti F, Magliocca FM and Citadini M: Fecal lactate and ulcerative colitis. Gastroenterology, 1988, 95:1564-1568. [ Links ]
48. Sandborn WJ: Pouchitis following ileal pouch-anal anastomosis: Definition, pathogenesis and treatment. Gastroenterology, 1994, 107:1856-1860. [ Links ]
49. Teixeira WGJ, Silva JH, Teixeira MG, Almeida M, Calache JE and Habr-Gama A: Pouchitis: extracolonic manifestation of ulcerative colitis? Rev Hosp Clin Fac Med S Paulo, 1999, 54:139-171. [ Links ]
50. Clausen MR, Tvede M and Mortensen PB: Short-chain fatty acids in pouch contents from patients with and without pouchitis after ileal pouch-anal anastomosis. Gastroenterology, 1992, 103:1144-1153. [ Links ]
51. Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Rossi SS, Hofman AF, Gores GJ and Phillips F: Fecal bile acids, short-chain fatty acids and bacteria after ileal pouch-anal anastomosis do not differ in patients with pouchitis. Dig Dis Sci, 1995, 40:1474-1483. [ Links ]
52. Sagar PM, Taylor FR, Godwin P, Holdsworth PJ, Johnston D, Lewis W, Miller A, Quirke P, Path MR and Williamson M: Acute pouchitis and deficiencies of fuel. Dis Colon Rectum, 1995, 38:488-493. [ Links ]
53. Nugent KP, Talbot IC and Phillips RK: Ulcerative colitis in familial adenomatous polyposis. Br J Surg, 1993, 80:254-257. [ Links ]
54. Campos FG, Waitzberg DL, Plopper C, Terra RM and Habr- Gama A: Ácidos graxos de cadeia curta e doenças colo-retais. Rev Bras Nutr Clin, 1998, 13:276-285. [ Links ]
55. Carpentier YA, Simoens C, Siderova V, Vanweyenberg V, Eggerickx D and Deckelbaum RJ: Recent developments in lipid emulsions: relevance to intensive care. Nutrition, 1997, 13 (suppl):73-78. [ Links ]
56. Alexander JW: Immunonutrition: the role of w-3 fatty acids. Nutrition, 1998, 14:627-633. [ Links ]
57. Morlion BJ, Torwesten E, Wrenger K, Puchstein C and Fürst P: What is the optimum w-3 to w-6 fatty acid ratio of parenteral lipid emulsions in postoperative trauma? Clin Nutr, 1997, 16:49. [ Links ]
58. Marsen TA, Pollok M, Oette K and Baldamus CA: Pharmacokinetics of omega-3-fatty acids during ingestions of fish oil preparations. Prostaglandins Leukot. Essent. Fatty Acids, 1992, 46:191-196. [ Links ]
59. Calder PC: Immunomodulatory and the anti-inflamatory effects of n-3 polyunsaturated fatty acids. Proc Nutr Soc, 1996, 55:737-774. [ Links ]
60. Grimm H, Schott J and Schwemmle K: Development of an immuno-neutral lipid emulsion for optimal postoperative management of intensive care patients. Langenbecks Arch Chir Kongressbd, 1998, 115:599-604. [ Links ]
61. Grimble RF: Nutritional modulation of cytokine biology. Nutrition, 1998, 14:634-640. [ Links ]
62. Shoda R, Matsueda K, Yamato S and Umeda N: Epidemiologic analysis of Crohn disease in Japan: increased dietary intak of n-6 polyunsaturated fatty acids and animal relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr, 1996, 63:741-745. [ Links ]
63. Almallah YZ, Richardson S, OHanrahan T, Mowat NA, Brunt PW, Sinclair TS, Ewen S, Heys SD and Eremin O: Distal procto-colitis, natural cytotoxicity and essential fatty acids. Am J Gastroenterol, 1998, 93:804-809. [ Links ]
64. Aslan AMD and Triadafilopoulos GMD: Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am J Gastroenterol, 1992, 87:432-437. [ Links ]
65. Hawkey CJ, Mahida YR and Hawthorne AB: Therapeutic interventions in gastrointestinal disease based on an understanding of inflammatory mediators. Agents Actions, 1992: 22-26. Spec Number. [ Links ]
66. Marotta F, Chui D, Safran P, Rezakovic I, Zhong G and Ideo G: Shark fin enriched diet prevents mucosal lipid abnormalities in experimental acute colitis. Digestion, 1995, 56:46-51. [ Links ]
67. Nieto N, Fernández MI, Torres MI, Ríos A, Suárez MD and Gil A: Dietary monounsaturated n-3 and n-6 long-chain polyunsaturated fatty acids affected cellular antioxidant defense system in rats with experimental ulcerative colitis induced by trinitrobenzene sulfonic acid. Dig Dis Sci, 1998, 43:2676-2687. [ Links ]
68. Hawthorne AB, Daneshmend TK, Hawkey CJ, Belluzi A, Everitt SJ, Holmes GKT, Malkison C, Shaheen MZ and Willars JE: Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 months radomized trial. Gut, 1992, 33:922-928. [ Links ]
69. McCall TB, OLeary D, BloomEield J and OMorain CA: Therapeutic potencial of fish oil in the treatment of ulcerative colitis. Aliment Pharmacol Ther, 1989, 3:415-424. [ Links ]
70. Salomon P, Kornbluth AA and Janowitz HD: Treatment of ulcerative colitis with fish oil n-3-w-fatty acid: an open trial. J Clin Gastroenterol, 1990, 12:157-161. [ Links ]
71. Lorenz R, Weber PC, Szimnau P, Heldwein W, Strasser T and Loeschke K: Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease - a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med, 1989, 225 (suppl):225-232. [ Links ]
72. Stenson WF, Cort D, Rodgers J, Burakoff R, DeSchryver- Kecskemeti K, Gramlich TL and Beeken W: Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med, 1992, 116:609-614. [ Links ]
73. Loeschke K, Ueberschaer B, Pietsch A, Gruber E, Ewe K, Wiebecke B, Heldwein W and Lorenz R: N-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig Dis Sci, 1996, 41:2087-2094. [ Links ]
74. Belluzzi A, Brignola C and Campieri M: Effects of an entericcoated fish oil preparation on relapses in Crohns disease. N Engl J Med, 1996, 334:1557-1616. [ Links ]
75. Lorenz-Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, Scheurlen C, Koop I, Pudel V and Carr L: Omega- 3 fatty acids and low carbohydrate diet for maintenance of remission in Crohns disease. A randomized controlled multicenter trial. Scand J Gastroenterol, 1996, 31:778-785. [ Links ]
76. Belluzzi A: Lipid treatment in inflammatory bowel disease. In: Bistrian BR, Walker-Smith JA (eds.): Nestle Nutr Workshop Ser Clin Perform Programme, 1999, 2:199-215. [ Links ]
77. Belluzzi A, Brignola C and Campieri M: Effects of a new fish oil derivate on fatty acid phospholipid-membrane pattern in a group of Crohns disease patients. Dig Dis Sci, 1994, 39:2589-2594. [ Links ]
78. Guarner F, Vilaseca J and Malagelada JR: Dietary manipulation in experimental inflammatory bowel disease. Agents Actions, 1992: 10-14. Special Conference Issue. [ Links ]
79. Shoda R, Matsueda K, Yamato S and Umeda N: Therapeutic efficacy of N-3 polyunsaturated fatty acid in experimental Crohns disease. J Gastroenterol, 1995, 8:98-101. [ Links ]
80. Geerling BJ, Badart-Smook A, van Deursen C, van Houwelingen AC, Russel MG, Stockbrugger RW and Brummer RJ: Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohns disease in remission: effects on antioxidant status and fatty acid profile. Inflamm Bowel Dis, 2000, 6:77-84. [ Links ]
81. Ikehata A, Hiwatashi N, Kinouchi Y, Yamazaki H, Kumagai Y, Ito K, Kayaba Y and Toyota T: Effect of intravenously infused eicosapentaenoic acid on the leukotriene generation in patients with active Crohns disease. Am J Clin Nutr, 1992, 56:938-942. [ Links ]
82. Grimminger F, Fuhrer D, Papavassilis C, Schlotzer E, Mayer K, Heuer K, Kiss L, Walmrath D, Kramer HJ and Seeger W: Influence of intravenous n-3 lipid supplementation on fatty acids profiles and lipid mediator generation in patients with severe ulcerative colitis. Eur J Clin Invest, 1993, 23:706-715. [ Links ]
83. Inui K, Fukuta Y, Kameda H, Kokuba Y and Sato M: The nutrition effect of a-linolenic acid-rich emulsion with total parenteral nutrition in a rat model with inflammatory bowel disease. Ann Nutr Metab, 1996, 40:227-233. [ Links ]
84. Fürst P and Kuhn KS: Fish oil emulsions: what benefits can they bring? Clinical Nutrition, 2000, 19:7-14. [ Links ]
85. Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N and Ito I: Effect of intravenous w-6 and w-3 fat emulsion on nitrogen retention and protein kinetics in burned rats. Nutrition, 1999, 15:135-139. [ Links ]
86. Campos FG: Efeitos de diferentes emulsões lipidicas parenterais na colite inflamatoria experimental. Tese de Doutorado apresentada à Faculdade de Medicina da Universidade de São Paulo, 1999. [ Links ]
87. MacDonald TT: Effector and regulatory Iymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol, 1999, 236:113-135. [ Links ]












 Curriculum ScienTI
Curriculum ScienTI