Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.20 no.2 Madrid mar./abr. 2005
Original
Iron metabolism, inflammation and anemia in critically ill patients.
A cross-sectional study
M. Muñoz, A. Romero*, M. Morales**, A. Campos*, J. A. García-Erce*** y G. Ramírez*
GIEMSA: Department of Biochemistry and Molecular Biology. School of Medicine. *Departments of Haematology and **Clinical
Biochemistry. University Hospital "Virgen de la Victoria". University of Málaga. Spain. ***Department of Haematology. University
Hospital "Miguel Servet". Zaragoza. Spain.
| Abstract Introduction: For critically patients, enteral immunonutrition results in notable reductions in infections and in length of stay in hospital, but not on mortality, raising the question as to whether this relate to the heterogeneous nature of critically ill patients or to the absence of the altered absorption of specific nutrients within the immunonutrient mix (e.g. iron). Immune-associated functional iron deficiency (FID) is not only one of the many causes or anaemia in the critically ill, but also a cause of inappropriate immune response, leading to a longer duration of episodes of systemic inflammatory response syndrome and poor outcome. (Nutr Hosp 2005, 20:115-120) Key words: Enteral route. Anemia. Iron. Inflammatory status.
| METABOLISMO DEL HIERRO, INFLAMACIÓN Y ANEMIA EN PACIENTES EN ESTADO CRÍTICO. UN ESTUDIO TRANSVERSAL Resumen Introducción: En los pacientes críticos, la inmuno-nutrición por vía enteral disminuye significativamente la tasa de infecciones y la estancia hospitalaria, pero no la mortalidad, por lo que se plantea la cuestión de sí esto es debido a la heterogeneidad de los pacientes críticos o a la ausencia o la absorción deficitaria de un nutriente específico en la mezcla administrada (p.e., hierro). La deficiencia funcional de hierro (DFH) causada por la inflamación no es sólo una de las causas de anemia en el paciente crítico, sino que también induce una respuesta inapropiada del sistema inmunitario, lo que origina una mayor duración de los episodios de respuesta inflamatoria sistémica y un peor pronóstico. (Nutr Hosp 2005, 20:115-120) Palabras clave: Vía enteral. Anemia. Hierro. Estado inflamatorio. |
Correspondence: Prof. Manuel Muñoz
GIEMSA. Facultad de Medicina
Universidad de Málaga
Campus de Teatinos, s/n.
29071 Málaga (Spain)
E-mail: mmunoz@uma.es
Recibido: 4-IV-2004.
Aceptado: 29-VI-2004.
Introduction
The potential to modulate the activity of the immune system by interventions with specific nutrients is termed immunonutrition, which has three potential targets, namely the mucosal barrier function, the cellular defence, and the local or systemic inflammation. This concept may be applied to any situation in which an altered supply of nutrients is used to modify inflammatory or immune responses. However, immunonutrition has become associated most closely with attempts to improve the clinical course of critically ill and surgical patients, who will often requiere an exogenous supply of nutrients through the parenteral or enteral routes1.
Four meta-analyses give a fairly consistent view of the clinical efficacy of enteral immunonutrition2-5. All four considered only randomised controlled trials in either surgical or critically ill patients and found that immunonutrition results in notable reductions in infections and in length of stay in hospital, but not on mortality. In general, the reduced infection rate and length of hospital stay are more pronounced in surgical than critically ill patients, and the reasons for these differences need to be investigated further in order to address whether these relate to the heterogeneous nature of critically ill patients or to the presence or absence of specific nutrients within the immunonutrient mix.
Critically ill patients are at greater risk of adverse outcomes than surgical patients. A biphasic response with an early hyperinflammatory response followed by an excessive compensatory response associated with immunosuppression is seen in many such patients. Therefore, early treatment needs to focus on decreasing the inflammatory response and prevent the compensatory immunosuppression.
Functional iron deficiency (FID) is a condition in which there is a decrease in iron available for metabolic processes and critically ill patients may develop FID in response to immune activation6. At laboratory level, FID is characterized by serum transferring saturation < 20%, serum ferritin < 100 ng/l, hypochronic red cells > 5%, or reticulocytes with low Hb content (CHr < 28 pg), in the presence of increased concentration of an inflammatory marker (e.g., C-reactive protein > 0.5 mg/dL)7. Research in animals and human beings has suggested that adequate iron scores are important not only for erythropoiesis but also for immune function and a deficiency may, therefore, predict patients with inappropriate immune responses8.
To this regard, Bellamy et al.9 found that duration of episodes of systemic inflammatory response syndrome and duration of stays at the intensive care unit were longer in critically ill patients with FID compared with patients without FID. This FID status can not be corrected by oral iron, since intestinal iron absorption is decreased in the presence of normal iron stores6. However, in a recent study, the administration of iron sucrose, alone or in combination with EPO, effectively reduced the requirements for allogeneic blood transfusion, and resulted in an amelioration of systemic inflammatory response and a reduction in mortality rate10.
Hence, functional iron status may be a marker of nutritional status or general health. Accordingly, this stud was initiated to determine the prevalence of FID in critically ill patients during their stay in intensive care in order to find the more appropriate population of patients that can benefit from iron therapy.
Material and methods
Study design
In this prospective cross-sectional observational study, we included adult medical or surgical patients admitted to the general intensive care unit (ICU) of a tertiary referral teaching hospital over 3 months. Patient's enrolment was perform every Monday, and only those patients with a ICU stay longer than 24 h were included (Length of ICU stay, LIS; min: 1 day; max: 38 days).
Blood samples
Venous blood samples for determination of haematimetric parameters were drawn in K2-EDTA (3 mL; VenoJet II, Terumo, Belgium), whereas those for determination of biochemical parameters were drawn in serum separator (4 mL; Sepacell, VenoJet II, Terumo, Belgium). All samples were collected at the laboratory, after daily routine analyses had been performed, and no extra blood samples were taken from any patient.
Laboratory parameters
Full blood cell counts, including reticulocytes (RETIC) (Pentra 120 Retic, ABX, France). Serum iron (SI, µg/dL), transferrin levels (TRF, mgdL) and saturation (satTRF, %) (Cobas Integra, Roche, Germany), ferritin (FRT, ng/mL), serum transferring receptor (sTfR, mg/dL) and C-reactive protein (CRP, mg/dL) (Image, Beckman-Coulter, USA) were measured in venous blood samples. Corrected RETIC counts for the degree of anaemia (reticulocyte index, RI) was calculated according to the expression: RI = % RETIC ´ (observed Htc/normal Htc) ´ 0.511.
Statistical analysis
All data are shown as the mean ± standard deviation (n) and an unpaired Student t test was used for comparison of means. We used the test r of Pearson to correlate values. All statistics were performed using Microsoft Excel 2000 and SPSS 11.0 packages (Licensed to the University of Málaga, Spain). A P value < 0.05 was considered statistically significant.
Results
Demographic and baseline characteristics of patients
A total of 131 patients were included in this observational cross-sectional study. Haematological and biochemical parameters were measured once by rescuing blood samples drawn for routine analyses. LIS at the moment of blood sample procurement varied between 1 and 38 days, with the following distribution: 57 (43.5%) out 131 were in the ICU for up to 3 days (Group A), 35 (26.7%) between 4 and 7 days (Group B), and 39 (29.9%) 8 days or more (Group C). Since patient enrolment were performed every Monday, we paid especial attention to avoid duplicate patient inclusion.
Mean age for the sample was 56 ± 17 years (range, 35-91 years). The majority of the patients were men (76%). Among surgical patients (50/131, 38%), the more frequently performed procedure was coronary artery bypass grafting, whereas sepsis and chronic obstructive pulmonary disease were the more frequent diagnostics among medical patients (81/131, 62%).
Haematologic parameters
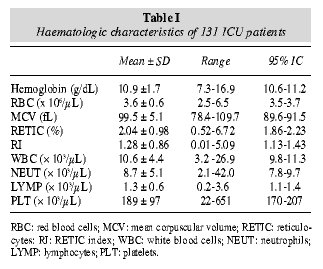
Mean values, ranges, and 95% CI of full blood count are summarised in table I. One hundred (76.3%) out of 131 patients were anaemic (Hb < 12 g/dL), with 43 (32.8%) presenting Hb levels below 10 g/dL. However, most of them presented normal MCV. RETIC counts were between 0.52 and 6.72%, with inadequate RETIC corrected counts in many cases. WBC counts were elevated in 75 (57%) with neutrophilia in 65 (50%), whereas 16 (12%) presented lymphocyte count below 800/µL (lymphopenia). Mean values of haematologic data after patient stratification according to LIS are summarised in table III.
Iron metabolism and inflammatory status
Iron metabolism status was assessed by measuring SI, TRF, satTRT, sTfR, and FTR (table II). Hypoferraemia (SI < 45 µg/dL) was observed in 90 (68.7%), satTRF < 20% in 69 (52.6%), sTfR > 1.9 mg/dL in 23 (17.6%) and > 2.3 in 17 (13%), FTR < 100 ng/mL in 30 (22.9%). The patient's inflammatory status was assessed by measuring serum levels of CRP (table II), which was found to be above the upper limit of normality (0.5 mg/dL) in 116 (88.1%) patients. Additional information was provided by WBC counts (table I) and TFR and FRT levels (table II). Mean values of iron metabolism parameters and CRP levels after patients stratification according to LIS are summarised in table III.
Correlations between inflammatory status and haematologic and iron parameters
Statistically significant positive and negative correlations (r of Pearson) were obtained for serum CRP levels and WBC, RBC, Hb, TRF, satTRF, and FRT, but not for CRP and sTfR (table IV, fig. 1). After stratification of patients according to LIS, most of these correlation remain significant for Groups A and B, whereas in Group C this was true only for SI (table III). There was also a strong correlation between TRF and FRT (-0.369; p < 0.01), but not between FRT and satTRF or SI. In the other hand, LIS correlated with Hb*, CRP**, TRF*, satTRF* and FRT**.
Fig. 1.- Correlation between levels of inflammatory marker (Log C-reactive protein)
and functional iron deficiency (% transferring saturation) in 131 ICU patients.
Discussion
Although there is no clear relationship between dietary iron intake and iron status, isotope studies have identified multiple dietary factors that influence iron absorption, susch as ascorbic acid, animal issue, phytates and polyphenols. The modern diet contains less red meta and is lower in iron than that consumed 30 years ago; however, there is no evidence to suggest that current dietary changes will have a major impact on iron status in the general population12.
In elderly populations, mean prevalence of folate (< 7 nmol/l) and vitamin B12 (< 148 pmol/l) deficiencies is high13,14, whereas iron deficiency use to be infrequent, although is more prevalent in women13,15. In these populations, prevalence of anaemia based in the WHO criteria (Hb < 13 g/dL in men; < 12 g/dL in women) has been reported to be 3-6%13,14, 16. On the contrary, subjects with inflammatory process had a higher prevalence of anemia (22.2% men, 31.6% women)13.
However, ICU patients show a completely different picture. Henche-Morilla et al.17 evaluated oligoelement and trace element levels when patients are admitted into the ICU and were included into a total parenteral nutrition (TPN) program. They found that, on admission to the unit, patients showed low serum levels iron, transferrin, zinc and calcium, and normal magnesium, phosphorus and copper figures. It is well known that major surgery, trauma and sepsis is followed by a systemic inflammatory response, whose humoral mediators (e.g., interleukin-1, interferon-g, and tumor necrosis factor-a) inhibit erythropoiesis both directly by suppressing erythroid colony growth and indirectly by suppressing erythropoietin production18. Most studies in critically ill adults focus on the longer stay patients and report an impaired EPO response to anaemia, based on comparison either with normal reference ranges or with a control group of patients with non-renal anaemia19-21. In contrast, one study of 10 patients with sepsis or septic shock in the first 4 days following admission demonstrated marked increases in EPO levels in those patients who subsequently died, paralleling changes in the acute phase response22. Similarly, Elliot et al.23 found that EPO levels are high in the first 48 hours of critical illness in patients with ARF, suggesting an acute renal response to injury. These patients also had the highest APACHE II scosres and mortality rates. EPO levels then decline over time, together with a reduction in haemoglobin concentrations, and by day 3 in the ICU the EPO levels for almost all patients are in the low normal range. This indicates failure of EPO effect rather than failure of production as the one of the mechanisms for the anaemia of critical illness.
In addition, these cytokines induce a functional iron deficiency (FID) status24,25; a clinical situation where the iron stores in the bone marrow macrophages is normal, but this iron is not available for erythropoiesis, due to an alteration of it release from the macrophages, of it incorporation to transferrin, or both6. Hepcidin, an hepatic acute phase protein recently discovered, seems to play a crucial role in these alterations26.
Most of our patients presented with a low serum iron, low transferrin level and saturation, and high ferritin: a pattern which is typical of the anaemia of chronic disease. These changes occur rapidly in acute as well as chronic illness as part of the systemic inflammatory response10. In fact, correlation between CRP and iron metabolism parameters were stronger in Group B (LIS: 4-7 days), and it is well known that effects of major surgery on iron metabolism are maximum during the first postoperative week25,26.
As expected, there was a strong negative correlation between TRF (a negative acute phase protein) and FRT (a positive acute phase protein) (-0.650; p < 0.01), but not between FRT and satTRF or SI, indicating that stored iron is not available. Thus, the high C-reactive protein combined with the low transferring saturation (fig. 1) and high ferritin support the view that, at least in part, the anaemia was a consequence of FID27, whereas only a small proportion of patients (13%) showed a real iron deficiency combined with inflammation, as suggested by high sTfR levels28. On average, the incidence of anaemia and the levels of Hb in our patients are similar to those recently reported for several European ICUs29.
However, FID leads not only to a blunted erythropoiesis but to an inappropriate immune response as well8. This might explain why systemic inflammatory response episodes last longer in critically ill patients with FID, with prolonged stay at the ICU and increased morbidity9. This FID status could not be corrected by oral iron, since intestinal iron absorption is inhibited, due to an hepcidin-induced reduction of its release through the enterocyte basolateral membrane26. On the contrary, once injected in vivo, the iron-carbohydrate complexes are metabolized, the iron is released where it then binds transferrin in the plasma, and the redundant carbohydrate moiety is then cleared via the liver30. Hence, the intravenous iron emerged as a therapeutic option for the treatment of FID in these patients, since the increased eryhtopoietic effect (4.5-5.5 times that of basal) of IV iron last 7 to 10 days, after which the iron is sequestered by the reticuloendothelial system31. In a recently published study the effectiveness of the administration of iron sucrose, alone or in combination with EPO, was assessed in a population of anemic critically ill patients10. Compared to those in the control group who only received folic acid, both treatments effectively reduced the requirements for ABT. In addition, patients treated with iron sucrose experienced an amelioration of systemic inflammatory response and a reduction in mortality rate. These beneficial effects were not as evident in patients receiving iron sucrose plus EPO, probably due to persistence of FID caused by the EPO-enhanced erythropoietic activity. Hence, it is possible that the correction of FID by administering iron sucrose has not only contributed to reduce the requirements for ABT by improving the erythropoietic response, but also to reduce postoperative morbi-mortality rate by restoring an adequate immune response.
Based on their findings, Henche-Morilla et al.17 arrived to the conclusion that the daily parenteral supplements of oligoelements should be higher than those recommended. We believe that this also applies to enteral nutrition, especially for those micronutrients, such as iron, for which intestinal absorption is limited by the systemic inflammation. From a theoretical point of view, our data suppor the idea that a significant amount of ICU patients might benefit from intravenous iron supplements, including those with enteral and parenteral nutrition. However, further research, preferably as a large randomised clinical trial, is needed to ascertain whether intravenous iron therapy might improved clinical outcomes in these patients.
Acknowledgement
This work has been partially supported by grant FIS PI 02/1826 from "Instituto de Salud Carlos III", Spain and the European Community, to Manuel Muñoz.
References
1. Culebras-Fernández JM, De Paz-Arias R, Jorquera-Plaza F, García de Lorenzo A: Nutrición en el paciente quirúrgico: inmunonutrición. Nutr Hosp 2001, 15:67-77. [ Links ]
2. Beale RJ, Bryg DJ, Bihari DJ: Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med 1999, 27:2799-805. [ Links ]
3. Heys SD, Walker LG, Smith I, Eremin O: Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer - a meta-analysis of randomized controlled clinical trials. Ann Surg 1999, 229:467-77. [ Links ]
4. Heyland DK, Novak F, Drover JW, Jain A, Su XY, Suchner U: Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001, 286:944-53. [ Links ]
5. Montejo JC, Zarazaga A, López-Martínez J, Urrutia G, Roque M, Blesa AL et al.: Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr 2003, 22:221-33. [ Links ]
6. Andrews NC: Disorders of iron metabolism. N Engl J Med 1999, 341:1986-95. [ Links ]
7. Thomas C, Thomas L: Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 2002, 48:1066-76. [ Links ]
8. Scrimshaw NS, Sangiovanni JP: Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr 1997, 66:464S-477S. [ Links ]
9. Bellamy MC, Gednaey JA: Unrecognised iron deficiency in critical illness. Lancet 1998, 352:1903. [ Links ]
10. Van Iperen CE, Gaillard CA, Kraaijehagen RJ, Braam BG, Marx JJ, Van de Wiel A: Response of eythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med 2000, 28:2773-8. [ Links ]
11. Erslev AJ: Reticulocyte enumeration. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ. Williams Hematology, 5th ed. New York: McGraw-Hill, 1995:L28. [ Links ]
12. Heath AL, Fairweather-Tait SJ: Clinical implications of changes in the modern diet: iron intake, absorption and status. Best Pract Res Clin Haematol 2002, 15:225-41. [ Links ]
13. Olivares M, Hertrampf E, Capurro MT, Wegner D: Prevalence of anemia in elderly subjects living at home: role of micronutrient deficiency and inflammation. Eur J Clin Nutr 2000, 54:834-9. [ Links ]
14. García Arias MT, Villarino Rodríguez A, García-Linares MC, Roncadio AM, García-Fernández MC; Iron, folate and vitamins B12 & C dietary intake of an elderly institutionalized population in Leon, Spain. Nutr Hosp 2003, 18:222-5. [ Links ]
15. De Groot CPGM, Van den Broek T, A Staveren W: Energy intake and micronutrient intake in elderly Europeans: seeking the minimum requirement in the SENECA study. Age Aging 1999, 28:469-74. [ Links ]
16. Charlton KE, Kruger M, Labadarios D, Wolmarans P, Aronson I: iron, folate and vitamin B12 status of an elderly South African population. Eur J Clin Nutr 1997, 51:424-30. [ Links ]
17. Henche Morilla AL, Romero Montero C, Llorente González C: Niveles de oligoelementos y elementos trazas en el momento de la admisión de los pacientes en las unidades de cuidados intensivos. Nutr Hosp 1990, 5:338-44. [ Links ]
18. Clemens J, Spivak JL: Serum immunoreactive erythropoietin during the perioperative period. Surgery 1994, 115:510-5. [ Links ]
19. Rogiers P, Zhang H, Leeman M, Nagler J, Neels H, Melot C et al.: Erythropoietin response is blunted in critically ill patients. Crit Care Med 1997, 23:159-62. [ Links ]
20. Von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU: Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med 1999, 27:2630-9. [ Links ]
21. Hobisch-Hagen P, Wiedermann F, Mayr A, Fries D, Jelkman W, Fuchs D et al.: Blunted erythropoietic response to aneamia in multiply traumatized patients. Crit Care Med 2001, 29:743-7. [ Links ]
22. Abel J, Spannbrucker N, Fandrey J, Jelkman W: Serum erythropoietin levels in patients with sepsis and septic shock. Eur J Haematol 1996, 57:359-63. [ Links ]
23. Elliot JM, Virankabutra T, Jones S, Tanudsintum S, Lipkin G, Todo S et al.: Erythropoietin mimics the acute phase response in critical illness. Crit Care 2003, 7:R35-40. [ Links ]
24. Biesma DH, Van de Wiel A, Beguin Y, Keraaijenhagen RJ, Marx JJM: Postoperative erythropoiesis is limited by the inflammatory effect of surgery on iron metabolism. Eur J Clin Invest 1995, 25:383-9. [ Links ]
25. Van Iperen CE, Kraaijenhagen RJ, Biesma DJ, Beguin Y, Marx JJM, Van de Wiel A: Iron metabolism and erythropoiesis after surgery. Br J Surg 1998, 85:41-5. [ Links ]
26. Brugnara C: Iron deficiency and erythropoiesis: new diagnostics approaches. Clin Chem 2003, 49:573-8. [ Links ]
27. Rodríguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG: Nutritional deficiencies and blunted erythropoietin response as cause of critical illness. J Crit Care 2001, 16:36-41. [ Links ]
28. Baillie FJ, Morrison AE, Fergus I: Soluble transferring receptor: a discriminating assay for iron deficiency. Clib Lab Haem 2003, 25:353-7. [ Links ]
29. Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A et al.: Anemia and blood transfusion in critically ill patients. JAMA 2002, 288:1499-507. [ Links ]
30. MacDougall IC: Intravenous administration of iron in epoetintreated haemodialysis patients-which drugs, which regimen? Nephrol Dial Transplant 2000, 15:1743-5. [ Links ]
31. Goodnough LT, Shander A, Spence R: Bloodless medicine: clinical care without allogeneic blood transfusion. Transfusion 2003, 43:668-76. [ Links ]