Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.21 no.1 Madrid ene./feb. 2006
ORIGINAL
Characteristics of glucose transport across the microvillous membranes
of human term placenta
Características del transporte de glucosa a través de las membranas con
microvellosidades de la placenta humana a término
Ravinderjit Kaur Anand** , Usha Kanwar** y Sankar Nath Sanyal*
*Department of Biophysics. **Department of Zoology, Panjab University, Chandigarh - 160014, India.
ABSTRACT
Transport characteristics of D-glucose were studied in the microvillous vesicles isolated from the human term placenta. Transport occurred by selective and rapid facilitated diffusion system which was inhibitable by phloretin and HgCl2. The transport was dependent on a transmembrane.
Na+-gradient indicating a "secondary active transport" system operating. The transport influx was saturable and the kinetic analysis based on Hanes-Woolf plot produced a kt and Jmax value of 1.2 mM and 34 nmoles. mgprotein-1.min-1, respectively. The efflux of D-glucose from the membrane vesicles in a pre-equilibrated assay conditions showed a distinct biphasic pattern differing significantly in the half time efflux. The t1/2 of the fast and slow components was found to be 15 sec and 660 sec, respectively. The transport showed distinct sensitivity to temperature and the Ea values both below and above the transition temperature of 37 ºC, as calculated from the Arrhenius plot were found to be 7600 and 5472 kCa1.mol-1, respectively. Inhibition studies with a number of sugars for hexose transport pathway showed that the glucose epimers, phosphorylated sugars, and even the disaccharides and the pentose sugars competed effectively with D-glucose. The influx was also inhibited by a number of steroids such as progesterone, 17α-hydroxyprogesterone, testosterone and estrogen. Insulin was found to increase glucose transport in a dose- dependent fashion at a concentration of 0.2-1 unit.ml-1. Ouabain, dinitrophenoi and nicotine strongly inhibited D-glucose uptake in the membrane vesicles.
Key words: Glucose transport. Brush border membrane. Placenta.
RESUMEN
Se estudiaron las características del transporte de la D-glucosa en las vesículas con microvellosidades aisladas de la placenta humana a término. El transporte ocurría por un sistema de difusión selectiva y facilitada rápida que podía inhibirse por floretina y por HgCl2. El transporte dependía de un gradiente de Na+ transmembrana,indicativo de un sistema operativo de "transporte activo secundario". El flujo de entrada del transporte era saturable y el análisis cinético basado en el gráfico de Hanes- Woolf produjo una kt y una Jmax de 1,2 mM y 34 nmoles• mg de proteína4•min-1, respectivamente. El flujo de salida de la D-glucosa desde las vesículas de membrana en condiciones de ensayo de pre-equilibrio mostró un patrón bifásico distintivo que difería significativamente en la mitad del flujo de salida. Se halló que la t1/2 de los componentes rápido y lento 15 seg. y 660 seg., respectivamente. El transporte mostró una sensibilidad distintiva a la temperatura, y se encontró que los valores de Ea, tanto por encima como por debajo de la temperatura de transición de 37º C, calculada por el gráfico de Arrhenius, fueron de 7.600 y 5.472 kCa1.mol-1, respectivamente. Los estudios de inhibición con una serie de azúcares para la ruta del transporte de la hexosa mostraron que los epímeros de la glucosa, los azúcares fosforilados, e incluso los disacáridos y los azúcares pentosa, competían de forma eficaz con la D-glucosa. El flujo de entrada también se inhibió por una serie de esteroides como la progesterona, la 17α-hidroxiprogesterona, la testosterona y los estrógenos. Se encontró que la insulina aumentaba el transporte de glucosa de manera dependiente de la dosis a una concentración de 0,2-1 unidades•ml-1. La ouabaína, el dinitrofenol y la nicotina inhibieron fuertemente la captación de D-glucosa por las vesículas de membrana.
Palabras clave: Transporte de glucosa. Membrana con borde en cepillo. Placenta.
Introduction
Placenta of various species has been shown to be selectively permeable to the D-isomer of glucose and related monosaccharides in the perfused tissue, and the transport is characterized by competitive inhibition and uphill movement by counterflow1. In vitro studies with the whole villous tissue have demonstrated that uptake of glucose into the syncytiotrophoblast is the initial step in the placental transfer2 which occurs both by diffusion and by a mediated sodium-dependent process conforming to the requirement of Michaelis- Menten kinetics3. However, the use of wholevillous tissue in the study of specific characteristics of placental transport is seriously limited by the presence of various cell types and subcellular membranes, while an isolated membrane preparation would clearly facilitate such investigation. The absorptive epithelial plasma membrane, called the microvilli, from the placental syncytium can be obtained in homogeneous preparation4. In further modification of the method, we have prepared closed vesicles capable of actively transporting amino acids and ions, while the purity of the membrane was established by electron micros-copy, marker enzyme assays, lipid compositional analysis and SDS-PAGE profile of the membrane proteins5- 8. In this article, we report investigations on Dglucose uptake which includes substrate specificity, kinetic properties of the transport, temperature dependence, effects of inhibitors and potential regulatory hormones and the sensitivity of the transport system to a number of membrane perturbing compounds. The transport in the isolated membrane vesicles which seemingly minimized the recapture of substrate from the medium9, also demonstrates the existence of a mediated pathway for exodus of the sugar.
Materials and Methods
Collection of placenta
Placentae were collected within 30 min of vaginal delivery from mothers with uncomplicated full-term pregnancies through the courtesy of the Government General Hospital, Sector-16, Chandigarh, on written request. Strict medical ethics was followed in obtaining the placentae and the patient's clinical history was checked from the antenatal Clinics record to avoid any pathological state such as the diabetes, hypertension and preeclampsia.
Placental microvillous (brush border) membrane preparation
The placenta was immediately placed on ice, brought to the laboratory within minutes and cut into pieces approximately 10-15 cm in diameter. Decidua and the chorionic plates were removed using a sharp microtome blade. The resulting villous tissue was washed with Earle's balanced salt solution to remove the blood10 which included 23 mM CaCl2, 1 mM Mg- SO4, 5.4 mM KCl, 116 mM NaCl, 5.6 mM D-glucose, 26 mM NaHCO3, 1 mM NaH2PO4 and 11 mg phenol red per liter of the solution. A 15-20 g placental tissue sample was spread out manually and then washed successively in ice- cold isotonic CaCl2 solution, in Earle's solution and in 40 mM Tris-HCl buffer, pH 7.4. These washings removed much of the blood from the intervillous spaces. The sample is briefly grounded with an electric food processor into small pieces of 1- 5 mm in diameter and placed in an ice-cold 0.9% NaCl solution. The pH of all solutions used in the preparation was adjusted to 7.4 and except where noted otherwise, all the steps were strictly performed at 4°C. They were then gently agitated at 4°C for 30 min using a magnetic stirrer. During this procedure the chorionic villi were spread out so that their surfaces could be well irrigated. The saline is poured off and centrifuged for 10 min at 800g in a refrigerated centrifuge (g forces are average value applied to the midpoint of the tube) to remove any fragments of tissues and further centrifuged at 10,000g for 10 min to remove the larger particles and intracellular debris. This is then followed by a high speed run of 100,000 g for 60 min in a Beckman ultracentrifuge (Model L8 80) using the Ti 60 fixed angle rotor, yielded a pale yellow jelly-like pellet. The brush border membrane (pellet) was further suspended in a 2 mM Tris-Hepes buffer by homogenization in an all glass hand homogenizer and repeatedly passing through the 23 gauge needle to give a final protein concentration of approximately 2 mg.ml -1. The isolated membrane was used for characterizations such as the transmission electron microscopy, marker enzyme analysis, SDS/PAGE of the membrane proteins, quantitative distribution of the lipids, as well as the studies on membrane transport.
Transport measurements
Measurement of glucose transport from the medium into the vesicles was performed under isotonic conditions. Membrane vesicles suspended in 2 mM Tris-Hepes buffer, pH 7.4 (buffer I), in the presence or absence of 130 mM NaCl were mixed with known concentration (1 mM) of 14 C-D-glucose in the same buffer with a sudden vortexing. The incubations were made at room temperature in a water bath and at indicated time interval as short as 3 sec, the transport was stopped by diluting the medium 10-fold with ice cold phloretin solution (0.2 mM in buffer I plus 2% ethanol). The vesicles were immediately separated from the substrate medium by rapid filtration under reduced pressure on mixed ester filters (0.45 µm pore size, 0.25 mm diameter) (Whatman Limited, Maidstone, England). The membrane vesicles were harvested on the filters pre-wet with dulled buffer I and on a syringe type filter holder (Whatman Limited, Maidstone, England). The vacuum for rapid filtration was generated with the help of a high capacity motor driven suction pump. The harvested vesicles were washed extensively 3-4 times with ice-cold buffer I. Non-specific binding of radioactivity to the filter in the absence of protein was determined by placing 100 µl of incubation solution (lacking vesicles) on the filter and by washing them as described. The radioactivity counts on such filter were accepted as the "filter blank". Each filter was suspended in scintillation cocktail containing 0.2% PPO, 0.04% POPOP, 6% naphthalene, 2% acetic acid, 2% ethylene glycol, 10% methanol and the rest 1,4 dioxan11. The radioactivity was determined by scintillation spectrometry using a Beckman (LS 7000) liquid scintillation counter.
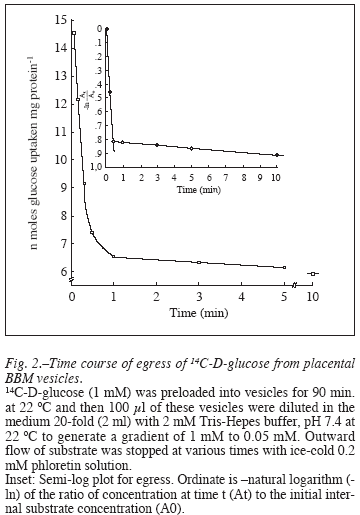
Measurement of transport of the same substrate in an outward direction or egress was performed according to the method of Johnson and Smith12. Vesicles were preloaded by equilibrating them for 1.5 h at 22°C with 14C-D-glucose ([S] =1 mM) in the presence or absence of 130 mM NaCl and were immersed in an ice bath thereafter. Aliquots of 100 µl each were warmed to 22°C and a large aliquot (2 ml) of buffer I was rapidly added to dilute the external substrate concentration to a low level and initiate egress. Phloretin as transport blocking agent (2 ml at 0°C) was added rapidly after a desired time interval to stop the egress and the vesicles were quickly recovered as above.
Measurement of membrane proteins
The amount of protein in the BBM surfaces was estimated by the modified sodium dodecyl sulphate (SDS)-Lowry procedure of Lees and Paxman13.
Results
Time course of D-glucose influx and egress in the placental BBM vesicles
Fig. 1 shows that in the presence of an inwardly directed sodium gradient (initial conditions: 130mM NaCl outside and zero inside), the influx of D-glucose in the placental BBM vesicles appeared to be extremely fast and follows a characteristic time dependent pattern. The quantity of the sugar in the vesicles reached a peak value of 32.17 nmoles.mg protein-1 at about 1 min which declined steadily thereafter and attained a near equilibrium value (steady state) at about 5 min. In the absence of Na+, the rate of uptake in the vesicles was much slower, never attained any peak accumulation and proceeded at a constant rate of about 20 nmoles.mg protein-1, which is only about 60% of the peak value as attained in the presence of Na+. The transient accumulation of the sugar in the vesicles suggests that the process is in some way linked to the inward movement of Na+ ions until that time when the vesicles were also equilibrated with the ion. In the subsequent experiments, the vesicles were incubated with the substrate for a period of 30 sec because it falls in the linear region of the uptake velocity.
Efflux of the substrate from the preloaded vesicles, called the 'egress' was equally fast with the internal concentration of the label falling to 75% in 20 sec and 30 sec in the presence and absence of NaCl, respectively (fig. 2). The rate then slowed abruptly as egress was studied for 10 min. The time course of egress was thus biphasic as clearly evident in the semi log plot (fig. 2 inset) that shows a halftime of 15 sec and 660 sec for the fast and slow components, respectively. Kinetic properties of the transport system were investigated with a range of substrate concentration of 1-30 mM and by measuring the initial rate of uptake of the label into the vesicles. The rate of D-glucose uptake was found to be concentration dependent over a range of 1-10 mM (fig. 3 a y b), which then saturates at higher concentrations of the monosaccharide assuming the hyperbolic kinetics typical of a protein mediated facilitated diffusion. In subsequent experiments, a substrate concentration of 30 mM was adopted ensuring a linear kinetics of the uptake process at a saturable substrate concentration. The data of uptake velocity (v) versus the substrate concentration ([S]) were transformed to a linear plot of HanesWoolf ([S]/v vs. [S]), and the apparent affinity constant of the uptake (kt) and the initial apparent maximum velocity (Jmax) were calculated from the x-intercept and the slope, respectively (fig. 3c). It follows the usual form of Michaelis-Menten equation for enzyme kinetics,
![]()
The uptake data plotted in this way produced a kt and Jmax value of 1.2 mM and 34.3 nmoleslmg protein/ min, respectively.
Inhibition by phloretin and HgCl2
Fig. 4 shows that the uptake of D-glucose in BBM vesicles was found to be inhibited by phloretin (60% inhibition) and H9Cl2 (30%). The time course of inhibition was also followed from 15 sec to 3 min, and while the "overshoot" in D-glucose uptake was once again visible, the inhibitory effects of phloretin and HgCl2 were clearly and uniformly discernible at all time points. Phloretin was therefore used as transport terminator in subsequent experiments to counter the rapidity with which the influx took place during the time lapse between incubation and filtration of membrane vesicles which was although as less as 1-2 sec.
The temperature dependence of uptake
The effect of temperature, if any, on the D-glucose uptake in the BBM vesicles was studied by following the uptake process at different temperatures ranging from 4-48 ºC (fig. 5). The results demonstrate that although the glucose uptake can be visualized even at lower temperatures, the optimum activity takes place at 37 ºC which considerably decreases thereafter at 48 ºC (25% inhibition). The uptake velocity at 0 ºC was only about 35% of that obtained at 37 ºC and at temperatures from 10-28 ºC, the uptake was found to increase linearly. The data was transformed to Arrhenius relationship of log maximum velocity to 1/T (absolute temperature) that yielded a non-linear curve (inset). From the plot were yielded the energy of activation (Ea) values below and above the transition temperature as calculated from the slope which were found to be 7600 and 5472 kCal.mol-1, respectively. The transition temperature (37 ºC) was directly recorded from the plot.
Substrate specificity
The substrate specificity for D-glucose transport in the placental BBM vesicles was determined by competitive inhibition with a number of mono- and disaccharides and their structural analogues when tested in a tenfold molar excess over the substrate in the incubation medium which also contained 130 mM NaC]. Table I shows that the D-glucose transport was inhibited substantially by the glucose analogue, D-galactose, deoxyglucose, D-mannose, pentose sugars like D-arabinose, D-xylose and the disaccharides such as a-lactose, maltose and sucrose. The phosphorylated sugars, glucose-1- phosphate, glucosc-6-phosphate and fructose 1,6-diphosphate also produced strong inhibition of D-glucose uptake in these vesicles. The inhibition constant (ki) as listed in the table was calculated following the equation:

The ki values showed, as listed in table I, that the disaccharides, phosphorylated sugars and arabinose with small ki values may prove to be the more potent inhibitors.
Effect of steroids
To study the effect of a number of steroid hormones on the uptake of D-glucose in the BBM vesicles, the membranes were preincubated for 10 min with a steroid and then the uptake studies carried out. During the transport, the media contained 500 µM steroid; 30 mM substrate and 130 mM NaCl. Progesterone, 17α-hydroxyprogesterone and testosterone strongly inhibited the uptake process and 13-estradiol inhibited moderately while cholesterol produced a rather weak inhibition (table II). Also, an amount of 5% ethanol present as solvent for the steroids had no effect on glucose uptake. The inhibition constant as listed showed that the hydroxylated progesterone with 0.22 mM was the most potent inhibitor.
Effect of insulin
Insulin was found to stimulate the glucose uptake in the placental BBM vesicles. Fig. 6 showed a dose dependent linear relationship of the increase in D-glucose uptake with an increment in insulin concentration in the incubation medium. It is also interesting to note that an extrapolation of the linear plot to the insulin concentration intersects the y-axis at a point which was identical to the Jmax value as obtained in the substrate kinetics experiment. The uptake values however, were obtained when BSA was present along with insulin, while without BSA, the value was about 29.5% less.
Effect of inhibitors
The uptake was inhibited by a number of compounds which are known membrane perturbing agents or metabolic inhibitors (fig. 7). Ouabain, a competitive inhibitor of Na+ /K+ -ATPase showed about 20% inhibition of glucose uptake. 2,4-dinitrophenol (DNP), a potent metabolic inhibitor blocked the influx to as much as 50% while its para substituent remained almost without any effect. Nicotine, a well documented drug of abuse, depressed the uptake significantly up to 18% of the control value.
Discussion
Glucose is a major fetal nutrient and understanding its transport across placenta is fundamental to the understanding of the fetal nutrition and metabolism. Since, the fetal liver is known to be deficient in gluconeogenic activities14, a transplacental movement of sugar from the maternal blood remained the only source for this primary energy molecule. Transport through the microvillous plasma membrane of the placental syncytiotrophoblast is believed to be the first step in the transfer process. The study of D-glucose uptake in the isolated membrane vesicles in the present investigation clearly demonstrates the presence of a high affinity glucose transport which is selective and mediated by a facilitated diffusion system. The process was saturable and had a kt of 1.2 mM which is similar to that reported in a number of tissues such as the adipocyte and the cultured fibroblasts from baby hamster kidney15. The results suggest that the monosaccharide is transiently accumulated within the vesicles by an electrogenic process dependent on the presence of an inward gradient of NaCl. The process is therefore, powered by sodium co-transport and aptly called as the "secondary active transport"16. The transport is characterized by a huge overshoot phenomenon which is also variously reported in 10 experiments with glucose and amino acid transport in the isolated microvillous membrane vesicles of similar transport epithelia, like intestinal villous and renal proximal tubule17,18. Outward flux of the monosaccharide is characterized by two distinct phases, a slower one preceded by an extremely rapid flux. The biphasic time course indicates the possibility of existence of two types of vesicular compartments with transport system of different affinity - one that fills and empties much faster than the other one. The transport also showed clearly its sensitivity towards temperature of incubation. The uptake was negligible at 4-15°C, proceeded maximally at 37°C and was inhibited thereafter, thus further suggesting that the process was transport protein-mediated and depended on the changes in membrane lipid microenvironment. The non-linearity in Arrhenius plot demonstrates a selective partitioning of the protein (transporter) in the ordered and liquid crystalline lipid phases. The break point as visualized directly from the plot corresponds to the membrane lipid phase transition (Tc). The Ea below the Tc (7600 kCal.mol-1) corresponded to the transition of the native molecule to the activated state while the Ea above the Tc (5472 kCal.mor1) may relate to denaturation or unfolding of the higher order structures.
The D-glucose uptake in placental BBM vesicles prefers substrates which are conformational analogues such as the D-galactose, D-mannose or the phosphorylated sugar at one or six position. It was interesting to note that a number of disaccharides also compete for the glucose binding sites. The possible explanation could be that these membranes are also richly endowed with disaccharide hydrolases19 which could provide the ready source, of the competing monosaccharide substrate. The transport system also seems to have little discrimination for the pentose sugar and the ketohexoses.
The microvillous membrane glucose transport system is inhibited by a number of steroid hormones which although are synthesized in vivo by the placenta and their local concentration cannot be told precisely, but may be presumed to be quite high. Smith and Brush20 reported a value of 1-2 µg of progesterone per g of isolated microvilli from placenta that corresponds to about 5 x 10-6 M which is within the range of the steroids where inhibition of glucose transport was observed in the present study. Direct inhibitory effects of steroids on glucose transport had been demonstrated in other cell types such as the diethylstilbestrol in erythrocytes21. It seems that the inhibition of glucose uptake in placental microvillous membranes from the maternal blood in ten-fold molar excess of the steroids is a part of the unique self regulatory mechanism of a tissue which is known to be autonomous. However, cholesterol, the precursor molecule for these steroids did have little effect on the uptake process. Steroid hormones have also been shown to inhibit strongly the uptake process of different amino acids in the placental membranes22. On the other hand, the transport process was stimulated in a dose dependent fashion by insulin. Placentalmembranes are richly endowed with high affinity insulin receptors23-25, although the biological effects of insulin on placenta are unknown and the membrane contains no hormone-sensitive adenylate cyclase26. Insulin however, seemed to inhibit the amino acid movement27. The inhibition is particularly pronounced in placental slice preparations28. Since, insulin is rapidly degraded by placental tissues24,29 we have added BSA to the incubation medium to slow down the proteolysis of insulin.
A number of drugs, membrane perturbing agents and metabolic inhibitors have been shown to inhibit the glucose uptake in the present study. Ouabain, a cardiac glycoside which specifically inhibits the Na+ /K+ ATPase by competitively binding with the K+ -inding sites of the enzyme30. The enzyme is responsible for generating the Na+ -gradient and therefore, the inhibitory effect of the drug on glucose transport which has to be cotransported with Na+, is also expected. Ouabain has also been found to inhibit the amino acid transport in placental22 and other epithelial surfaces31. Similar compounds like digoxin and ethacrynic acid which inhibited the transport ATPase, also strongly blocked the amino acid and glucose transport in placental membranes32. The oxidative inhibitor, dinitrophenol inhibited the uptake while in the presence of p-nitrophenyl phosphate, the uptake of glucose appeared to maintain the near normal rate. The results are similar to that of the glucose and amino acid uptake in placental slice experiments32. Dinitrophenol is also reported to inhibit the vitamin B12 uptake in placental membranes33.
The uptake is also inhibited drastically by metabolic poison, HgCl2 and also by phloretin. Phloretin has some structural similarities to the steroids and is also known to be weakly estrogenic34. It has an o-β-D-glucopyra-nosine in its molecule, which is used experimentally to produce glycosuria and is a potent inhibitor of glucose transport in the intestinal and renal membrane vesicles35,36. Nicotine is a major drug of abuse, known to depress the placental amino acid transport37 and may be one of the factors that cause reduction in intrauterine fetal growth in smoking mothers38. Nicotine considerably inhibited the glucose uptake which is surprising in view of the fact that placenta is devoid of any kind of nerve. However, it is known that placenta is rich in acetyl choline and its metabolizing enzymes39. Nicotine binds to the acetyl choline/muscarinic receptor and it is believed that cholinergic regulation may form the basis of glucose and amino acid transport in placenta37.
References
1. Johnson LW, Smith CH: Glucose transport across the basal plasma membrane of human placental syncytiotrophoblast. Biochim Biophys Acta 1985, 815: 44-50. [ Links ] [ Links ]
3. Stacey TE, Weedon AP, Haworth C, Ward RHT, Boyd RDH: Fetomatemal transfer of glucose analogues by sheep placenta. Am J Physiol 1978, 234: E32-E37. [ Links ]
4. Smith NC, Brush MG, Luckett S: Preparation of human placental villous surface membrane. Nature 1974, 252: 302-303. [ Links ]
5. Anand RJK, Kanwar U, Sanyal SN: Isolation and characterization of the basal cell membranes of the human term placenta. Indian J Expt Biol 1996a, 33: 298-307. [ Links ]
6. Anand RJK, Kanwar U, Sanyal SN: Ca 2+ - transport across brush border and basal surfaces of human term placenta. Indian J Expt Biol 1996b, 34: 786-793. [ Links ]
7. Anand RJK, Kanwar U, Sanyal SN: Placental membrane transport. Leucine transport across the brush border and basal cell membrane surfaces. Res Expt Med 1996c, 196:29-43. [ Links ]
8. Anand RJK, Kanwar U, Sanyal SN: Transport of glycine in the brush border and basal cell membrane vesicles of the human term placenta. Biochem Mol Biol Intn 1996d, 38(1): 21-30. [ Links ]
9. Smith CH, Nelson DM, King BF, Donohue TM, Ruzycki SM, Kelley LK: Characterization of a microvillous membrane preparation from human placental syncytiotrophoblast, a morphologic, biochemical, and physiologic study. Am J Obstet Gynecol 1977, 128: 190-197. [ Links ]
10. Paul J. In: Cell and Tissue Culture. Williams & Wilkins, Baltimore, 1965, p. 83. [ Links ]
11. Butler FE: Determination of tritium in water and urine. Liquid scintillation counting and rate of drift determination. Anal Chem 1961, 33: 409-413. [ Links ]
12. Johnson LW. Smith CH: Monosaccharide transport across microvillous membrane of human placenta. Am J Physiol 1980, 238: C160-C168. [ Links ]
13. Lees M, Paxman S: Modification of the Lowry procedure for the analysis of proteolipid protein. Anal Biochem 1972, 47: 184-194. [ Links ]
14. Kalhan SC, D' Angelo LJ, Savin SM, Adam P AJ: Glucose production in pregnant women at term gestation: Sources of glucose for human fetus. J Clin Invest 1979, 63:388-394. [ Links ]
15. Eilam Y, Stein WD: Kinetic studies of transport across red blood cell membranes. In: Methods in Membrane Biology, (E.D. Kom, ed.) Plenum Publishing Corporation, New York, 1974, Vol. 2, pp.283. [ Links ]
16. Boyd CAR, Lund EK: L. Proline transport by brush border membrane vesicles prepared from human placenta. J Physiol 1981, 315: 9-19. [ Links ]
17. Ghishan FK, Sutter W, Said H, Leonard D, Pietsch J, Abumrad N: Glutamine transport by rat basolateral membrane vesicles. Biochim Biophys Acta 1989, 979: 77-81. [ Links ]
18. Inui K, Okano T, Takano M, Kitazawa S, Hori R: A simple method for the isolation of basolateral plasma membrane vesicles from rat kidney cortex. Biochim Biophys Acta 1981, 647: 150-154. [ Links ]
19. Ramaswamy D, Malathi P, Crane RK: Demonstration of hydrolase related glucose transport in brush border membrane vesicles prepared from guinea pig intestine. Biochem Biophys Res Commun 1976, 68: 162-168. [ Links ]
20. Smith NC, Brush MG: Preparation and characterization of human syncytiotrophoblast plasma membrane. Medical BioI 1978, 56: 272-276. [ Links ]
21. LeFevre PG: Molecular structural factors in competitive inhibition of sugar transport. Science 1959, 130: 104-105. [ Links ]
22. Sybulski S, Tremblay PC: Uptake and incorporation into protein of radioactive glycine by human placentae in vitro. Am J Obstet Gynccol 1967, 97: 1111-1118. [ Links ]
23. Nelson DM, Smith RM, Jarett L: Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes 1978, 27:530-538. [ Links ]
24. Steel RB, Mostey JD, Smith CH: Insulin and placenta: degradation and stabilization, binding to microvillous membrane receptors, and amino acid uptake. Am J Obstet Gynecol 1979, 135: 522-529. [ Links ]
25. Whitsett JA, Lessard JL: Characteristics of the microvillous brush border of human placenta: insulin receptor localization in brush border membranes. Endocrinology 1978, 103: 1458-1468. [ Links ]
26. Whitsett JA, Johnson CL, Howkins K: Differences in localization of insulin receptors and adenylate cyclase in the human placenta. Am J Obstet Gynecol 1979, 133: 204-207. [ Links ]
27. Shotwell MA, Kilberg MS, Oxender DL: The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta 1983, 737: 267-284. [ Links ]
28. Dancis J, Money WL, Springer D, Levitz M: Transport of amino acids by placenta. Am J Obstet Gynecol 1968, 101: 820+829. [ Links ]
29. Posner, B.I: Insulin receptors in human and animal placental tissue. Diabetes 1974, 23: 209-217. [ Links ]
30. Yudilevich DL, Sweiry JH: Transport of amino acids in the placenta. Biochim Biophys Acta 1985, 822: 169-201. [ Links ]
31. Miller RK, Bemdt WO: Mechanisms of transport across the placenta: An in vitro approach. Life Sci 1975, 16: 7-30. [ Links ]
32. Garrett RJB, Garreett NE: Archdeacon JW. Binding of iron by rat placental tissues. Experientia 1973, 29(4): 463-464. [ Links ]
33. Dodds EC, Lawson W: Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc Royal Soc London Ser BV 1938, 125: 222-232. [ Links ]
34. Atisook K, Carlson S, Madara JL: Effects of phlorizin and sodium on glucose elicited alterations of cell junctions in intestinal epithelia. Am J Physiol 1990, 258: C77-C85. [ Links ]
35. Semenza G, Kessler M, Hosang M, Weber J, Schmidt U: Biochemistry of the Na + , D-glucose cotransporter of the small intestinal brush border membrane. Biochim Biophys Acta 1984, 779: 343-379. [ Links ]
36. Sastry BVR. Placental toxicology: Tobacco smoke, abused drugs, multiple chemical interactions and placental function. Reprod Fertil Dev 1991, 3: 355-372. [ Links ]
37. Hasselmeyer EG, Meyer MB, Catz C, Longo LD: Pregnancy and infant health. In: Smoking and Health: A report of the Surgeon General (JM Pinney, ed.) US Department of Health, Education and Welfare, Washington DC; Publication No. (PHS) 79-5006, 1979, p.8-1to 8-93. [ Links ]
38. Sastry BVR. Olubadewo J, Boehm FH: Effects of nicotine and cocaine on the release of acetylcholine from isolated placental villi. Arch lnt Pharmacodyn Ther 1977, 229: 23-26. [ Links ]
39. Sastry BVR. Bishop MR, Janson VE, Ochillo RF: Relationship between acetylcholine and maturation of human placenta. Pharmacologist 1978, 20(3): 202. [ Links ]
![]() Correspondencia:
Correspondencia:
Dr. S. N. Sanyal
Department of Biophysics
Panjab University, Chandigarh- 160 014, INDIA
Tel: +91-172-2534119
E-mail: sanyalpu@yahoo.co.in; sanyal@pu.ac.in
Recibido: 28-IV-2005.
Aceptado: 14-VI-2005.