Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.23 no.3 Madrid may./jun. 2008
Design and validation of a scale to assess preferences of type 2 diabetic patients towards different nutritional supplements
Diseño y validación de una escala para evaluar las preferencias de pacientes diabéticos tipo 2 de los diferentes suplementos nutritivos
M. A. Rubio*, J. López Arrieta**, M. Ruiz***, J. Garrido***, J. A. Rubio****, J. del Llano*****, C. Casimiro****** y F. Raigada*****
*Servicio Endocrinología. Unidad de Nutrición. Hospital Clínico Universitario San Carlos. Madrid.
**Servicio Geriatría. Hospital General Universitario Gregorio Marañón (Cantoblanco). Madrid.
***Departamento de Psicología Social y Metodología. Facultad de Psicología. Universidad Autónoma de Madrid. Madrid.
****Servicio de Endocrinología y Nutrición. Hospital Príncipe de Asturias. Alcalá de Henares. Madrid.
*****Fundación Gaspar Casal. Madrid.
******Departamento Médico. Abbott Laboratories. Spain.
Dirección para correspondencia
RESUMEN
Objetivos: Diseñar y validar una escala (Escala de Madrid) para evaluar las preferencias de pacientes diabéticos tipo 2 de los diferentes suplementos nutritivos y descubrir aquellos atributos del sabor que son más discriminatorios.
Contexto: pacientes ambulatorios con diabetes mellitus tipo 2.
Materiales y métodos: 18 controles y 106 pacientes con diabetes tipo 2 recibieron 2 de los 7 estímulos estudiados (6 suplementos nutritivos y un estímulo salado diferencial) y posteriormente completaron ambas escalas y una pregunta criterio. Dos semanas después, se volvió a pasar el cuestionario a 30 pacientes diabéticos. Se estudiaron las propiedades psicométricas de la escala de Madrid y se evaluó la importancia relativa de cada atributo de los estímulos.
Resultados: Realización: La Escala de Madrid comprende 8 preguntas y se completa en menos de 5 minutos; Dimensionalidad: una única dimensión que explica el 45,1% de la varianza. Fiabilidad: Alfa de Cronbach 0,806; coeficiente de correlación intra-clase 0,835 (intervalo de confianza al 95%: 0,653-0,922). Validez concurrente: índices de correlación para la puntuación total corregida con la pregunta criterio y la escala modificada de degustación de vinos, 0,731 (p < 0,0005) y 0,774 (p < 0,0005), respectivamente. La escala discriminaba entre los individuos menores y mayores de 75 años, y entre los suplementos y su estímulo diferencial. Preferencias: Glucerna SR® chocolate, Glucerna SR® fresa, Glucerna SR® vainilla, Diasip® vainilla, Clinutren® vainilla y Resource diabet® vainilla.
Conclusión: La escala de Madrid posee propiedades psicométricas adecuadas para su uso en investigación y en la práctica clínica diaria.
Palabras clave: Diabetes mellitus. Preferencias. Suplementos nutritivos. Escala dimensional.
ABSTRACT
Objectives: To design and validate a scale to evaluate preferences of type 2 diabetic patients towards nutritional supplements (Madrid scale) and to discover those taste attributes that are more discriminating.
Context: ambulatory patients with type 2 diabetes mellitus.
Materials and methods: 18 controls and 106 type 2 diabetic patients received 2 of the 7 stimuli studied (6 nutritional supplements and a differential salty stimulus) and then completed both scales and a criterion question. Two weeks later, 30 diabetic patients received a retest. The psychometric properties of the Madrid scale were studied and the relative importance of each stimuli attribute was assessed.
Results: Feasibility: The Madrid scale consists of 8 questions and is completed in less than five minutes; Dimensionality: A single dimension which explains 45.1% of the variance. Reliability: Cronbach's , 0.806; intraclass correlation coefficient, 0.835 (95% confidence interval: 0.653-0.922). Concurrent validity: Correlation indexes of the corrected total score with the criterion question and the Modified Wine-Tasting Scale, 0.731 (p < 0.0005) and 0.774 (p < 0.0005), respectively. The scale discriminated between subjects younger and older than 75 years and between supplements and the differential stimulus. Preferences: Glucerna SR® chocolate, Glucerna SR® strawberry, Glucerna SR® vanilla, Diasip® vanilla, Clinutren®vanilla and Resource diabet® vanilla.
Conclusion: The Madrid scale has adequate psychometric properties for its use in research and daily clinical practice.
Key words: Diabetes mellitus. Preferences. Nutritional supplements. Dimensional scaling.
Introduction
Malnutrition is a common problem in patients admitted to the hospital, particularly in elderly patients, where the reduction of muscle mass and increased protein catabolism are common phenomena associated with metabolic stress,1 and which frequently require the addition of nutritional supplements to the diet.2Standard formulas may not meet the specific needs of diabetic patients and may cause undesirable increases in blood glucose levels.3 As a result, different formulations have been developed for patients with diabetes, most of which meet the recommendations of the European Association for the Study of Diabetes and the American Diabetes Association.4 However, successful compliance with prescription of oral supplements depends largely on their acceptance by patients, and it has been suggested that each patient could perform a taste test to determine his/her preferences, thus leading to individualized therapy.5 The large number of commercially available preparations and flavours increases the chance of finding an appropriate product for each patient, but it also presents obvious logistic problems. It is perhaps because of this that most of the studies carried out to determine the preference of diabetic patients have used a relatively small number of products.
Physicians, nurses and dieticians tend to develop their own opinions on which products are best accepted by patients. However, this process is very subjective and the validity of the choices made is questionable, to the point that nutritional therapy may become a source of conflict between physicians and their patients.6 Therefore, it is appropriate that specific tools be available to determine the preferences of diabetic patients. Amerine and Singleton developed the Wine-Tasting Scale7 in 1977, which was subsequently adapted for its use with nutritional supplements for cancer patients.5 This scale has been translated and subsequently used in the Spanish population,8, 9 but its psychometric properties have not been clearly determined. We therefore proposed to develop a specific scale, which we have called the Madrid scale, which, using the Modified Wine-Tasting Scale as a basis, adds all the items considered relevant in the opinion of specialists and diabetic patients to determine preference for a nutritional supplement. In addition, it was proposed to apply the scale to a wide range of commercial preparations and flavours to allow patient preferences to be ordered.
Materials and methods
Patients
Patients of both sexes, diagnosed of type 2 diabetes mellitus (DM2) were enrolled at the Departments of Endocrinology and Geriatrics of two university hospitals. The study protocol was approved by the local Ethics Committee and patients were consecutively recruited from those giving their informed consent. Inclusion criteria were: patients clinically stable, with a glycosylated haemoglobin value (HbA1c) < 7.5%, with 50 or more years of age and able to understand and respond to the questionnaire. Exclusion criteria were: presence of microalbuminuria or advanced retinopathy, patients with previous or current neoplastic disease, a history of chronic renal failure or chronic liver disease, clinical or radiological gastroparesis or an aversion to the flavour vanilla (the anchor stimulus) were excluded. For the study of the psychometric properties of the scale, a small group of eighteen nondiabetic patients was recruited in the same centres and with the same criteria except the diagnosis of DM2 (control group). Table 1 shows the main clinical characteristics of diabetic and non diabetic subjects.
Stimuli and sampling method
For the study of the psychometric properties of the Madrid scale, six sweet enteral nutritional supplements were used: Glucerna SR® (Abbott Laboratories) strawberry, vanilla and chocolate flavours; Resource Diabet® (Novartis) vanilla flavour; Clinutren Diabetes®(Nestlé) vanilla flavour; Diasip® (Nutricia) vanilla flavour. Finally, a differential stimulus, a typical cold tomato soup of similar density to enteral supplements called gazpacho was used (a commercial brand Alvalle® was chosen for reasons of it's know flavour stability).
An incomplete fractional design was used in which each patient received two stimuli, one of them (Glucerna SR® vanilla) was used as anchor stimulus to establish comparisons. The two stimuli were presented in a volume of 50 cc, served in covered cups at room temperature, separated by approximately 15 minutes and drinking a glass of water between the two presentations. The order of stimuli presentation was randomized. The questionnaires were completed immediately after exposure to the stimulus.
Presenting a large number of stimuli to the same patient entails serious drawbacks. First, non trained patients get tired and do not perform the task accurately. Second, patients get full and are not able to taste more than 2-3 stimuli. Third, it could be difficult to discriminate between more than two possibly similar stimuli. Given this limitation, an incomplete sample design was used in order to cover the maximum range of possibilities by means of paired comparisons accomplished by sub-samples of different patients all of them linked by an anchor stimulus. The design was set up to compare, in one hand all available vanillas, and in the other hand all flavours of a same brand (Glucerna) plus gazpacho. Each product was compared with the anchor stimulus at least by 7 DM2 patients. This kind of design has been successfully used in the estimate of population health state preferences.10, 11
Questionnaires
The Madrid scale, the Modified Wine-Tasting Scale and the criterion question were administered.
The Madrid scale consists of 8 questions referring to 8 attributes of the nutritional supplements: Appearance, Smell, impression in the mouth (Texture), taste, sensation of fullness (Fullness), Sweetness, taste after swallowing (Aftertaste), and overall impression (Impression). The 8 questions were presented as multiple-choice questions with 3 mutually exclusive ordered response categories. Response categories could be assumed to constitute Likert scales. In order to assign initial correction scores to each category, attribute categories were ranked from 1 (less desirable) to 3 (more desirable) by an expert panel. Appendix 1 shows the consensus score assigned to each attribute response category (only shows in spanish version). Attribute scores were assumed to be additive in order to obtain a single raw overall score, which would attain values between 8 (lowest preference) and 24 (highest preference) points. The raw overall score was transformed to a 0-100 scale, so that the corrected total score (CTS) represents the percentage of the highest possible preference assigned by a given subject to a given stimulus (see Appendix 1).
The Modified Wine-Tasting Scale was developed in the United States5 and has been used in Spanish diabetic patients by various authors.8, 9 It consists of 6 attributes: Appearance, Body, Taste, Aroma, Sweetness, and Aftertaste. The overall score of the scale quantifies the acceptance of the stimulus by a given subject in a 0-20 scale.
The criterion question, "What did you think of the taste of the drink?", was presented accompanied by a 10-point visual analogue scale, where 0 represented the worst acceptance (I don't like it at all), and 10 the best acceptance (I like it a lot).
Study design
Development of the Madrid scale consisted of four successive phases:
1. Content determination: A panel of experts reviewed the Modified Wine-Tasting Scale8 and suggested some changes based on their clinical experience. Two discussion focus-groups (of 10 DM2 patients each), were consulted to determine relevant aspects to be included in the new scale. Both sources of information were considered and a first version of the scale was built.
2. Pilot study: The first version of the Madrid scale was administered to a small sample of 25 patients with DM2 to assess its feasibility: administration time, comprehension, and possible idiomatic expressions with different cultural acceptance. Wording was adapted by the expert panel when needed, and changes were included in the scale final version.
3. Test: The scale was administered to a sample of 106 diabetic patients and 18 controls to determine the psychometric properties of the scale: reliability and validity. During the first session (test) demographic data were collected and the two stimuli were presented as described above. After each stimulus the patient was asked to complete the Madrid scale, the Modified Wine-Tasting Scale and the criterion question, with the aid of a nurse.
4. Retest: A second administration (retest) was accomplished 15 days after the baseline measure to determine the temporal stability of measurements. A sub-sample of 30 patients was used. Patients received a single stimulus, identical to the first one presented in the test session, and were asked to complete the Madrid scale. The retest was administered only to diabetic patients.
Data analysis
Quantitative variables are expressed as mean ± standard deviation (SD). Qualitative variables are presented as absolute frequencies and/or percentages.
Feasibility. Time for completion of the Madrid scale and the percentage of unanswered questions were calculated. Ceiling and floor effects were considered if more than 50% of patients chose the maximum or minimum response category. Completion time was considered acceptable at an average of 10 minutes or less.
Dimensionality. A metric estimate of the Madrid scale dimensionality was obtained using Exploratory Factor Analysis through Principal Components extraction, with Varimax and Promax rotations. The number of factors was determined using the K1 rule12, 13 and the Screen Test.14, 15
Reliability. Internal consistency was evaluated using Cronbach's . Test-retest stability was evaluated by between-forms Pearson correlation index, t-test for paired data, and Intraclass Correlation Coefficient (ICC). In order to ensure independence of measures,only one of the two measurements obtained in the test session was used in the analyses of internal consistency and dimensionality.
Validity. Concurrent validity was assessed by means of Pearson correlation between the Madrid scale, the Modified Wine-Tasting Scale, and the criterion question. Item analysis of responses to items of the Madrid scale and those of the Modified Wine-Tasting Scale was also carried out. Construct validity was inferred from the discrimination performance of the scale between groups of patients with different known characteristics with the same stimulus presented. Mann-Witney U test was used. Since diabetic patients have an altered sense of taste,15-19 controls and diabetic patients were compared. Moreover, alteration of the sense of taste and smell has also been reported with age.20 Thus patients < 75 and > 75 years of age were also compared. Discrimination between different stimuli was tested with Kruskal-Wallis test and Mann-Witney U test, with Bonferroni's correction, to determine pairs of stimuli that differed from each other.
Scaling: Multidimensional scaling was used to compare the stimuli without the need to assume ordinality. Since the expert panel questioned the existence even distance between the response categories and even the possible order of categories, non metric techniques where used to obtain optimal scale free quantifications for response categories within each attribute.21 It was also pursued to establish the order of preference of the flavours that were presented to the subjects, and determine the taste attributes with highest discriminating capacity. Optimal scaling regression models22 were used. Two analyses were performed, the first comparing the most dissimilar stimulus, gazpacho, with all nutritional supplements grouped, and the second comparing the supplements with each other.
All comparisons were two-tailed and a p-value< 0.05 was considered significant, except in the multiple comparisons. All analyses were performed with the SPSS statistical package version 12.05.
Results
In the first phase of development of the Madrid scale, the group of experts did add two new attributes,"sensation of fullness" and "taste", to the Modified Wine-Tasting Scale to prepare the preliminary version of the Madrid scale. All attributes received the same weight to obtain the total additive final score. Discussion groups did not add any new attribute, but did modify some of the response categories to improve comprehension.
In the second phase of study, the questionnaire was presented to the pilot group whose characteristics are given in Table I. Time for completion of the provisional Madrid scale was less than 5 minutes in all cases. No comprehension problems where found. Considering these results, the questionnaire was adopted as the final Madrid scale (see Appendix 1).
Feasibility
The 248 administrations of the Madrid scale performed during the test were examined. Time for completion was less than 5 minutes in all cases, regardless of whether it was the first or second administration of the scale, and all 8 questions were answered in the 248 administrations. A ceiling effect was observed for the attributes "Appearance", "Texture", "Taste" and "Aftertaste", which was independent of the stimulus presented, while a ceiling effect was found or not for the attributes "Smell", "Fullness" and "Opinion" depending on the stimulus considered. For the attribute "Sweetness", a ceiling effect was found for some supplements and a floor effect was found with gazpacho.
Dimensionality
In this analysis 124 independent measurements corresponding to one of the two stimuli presented in the test were used. The randomly chosen stimuli were: Glucerna vanilla, 61 presentations; Glucerna strawberry, 16 presentations, Glucerna chocolate, 7 presentations; Resource vanilla, 11 presentations; Clinutren vanilla, 13 presentations; Diasip vanilla, 7 presentations; gazpacho, 9 presentations. The exploratory factor analysis found a single factor with an Eigen value greater than 1 (3,612), explaining 45.1% of the total variance. The communalities found (i.e., the proportion of the variance of each question explained by the factor solution) varied from 0.162 to 0.774. Correlations between the questions and the factor extracted varied from 0.402 to 0.880. Solutions of higher dimensionality were explored but were not interpretable.
Reliability
The corrected total score (CTS) of the Madrid scale for the vanilla anchor stimulus ranged from 0.125 to 100, with a mean value of 78.92 ± 18.91. The CTS distribution is uni-modal and presents a negative bias (Skewness = -1.207, SE = 0.223).
Item correlations ranged from 0.067 (Sweetness-Appearance) to 0.763 (Aftertaste-Opinion). All item correlations were positive. Cronbach's reliability coefficient for the Madrid scale attained a value of 0.806.
No significant difference was found (t = 1,144; df= 29; p = 0.262) between the mean CTS at test (79.2 ± 22.7), and retest (75.6 ± 22.3) measurements. Correlation between test and retest was 0.717 (p < 0.0001). The average ICC for the eight items scale was statistically significant (p < 0.0005) with a value of 0.835 (95% CI: 0.653-0.922).
Validity
Content validity was assured by respecting the contents of the original scale of reference, supervision by the expert panel and consideration of the focus-groups opinions. Structure-Construct validity was supported by the exploratory factor analysis, which indicated a one-dimensional solution encompassing all attributes.
The correlation index between the CTS score of the Madrid scale and the overall score of the Modified Wine-Tasting Scale was 0.731 (p < 0.0005) and between CTS and the score in the criterion 0.774 (p < 0.0005). The correlations between both scales single items are presented in table II. Four out of the 5 conceptually matching attributes (Appearance, Aroma, Sweetness and Aftertaste), obtained the highest correlation with the equivalent question of the concurrent scale, while the attribute "Taste" achieved high correlation also with some other attributes. In fact it attained significant correlations with all other attributes of the concurrent scale.
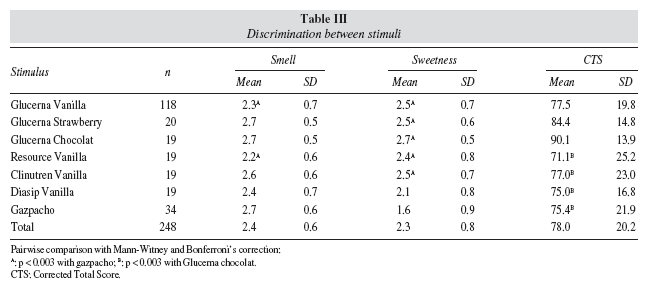
Comparing groups, the attribute "Sweetness" of the Madrid scale tended to discriminate between controls (n = 18, score 1.3 ± 0.7) and diabetics (n = 16, score 1.9± 1.0; p = 0,065) when the stimulus was gazpacho, but no significant differences were found for Glucerna SR vanilla stimulus in any of the 8 attributes or in the CTS. Comparing age groups, significant differences were found in the attribute "Smell" for Glucerna SR vanilla and gazpacho stimuli, and in the attributes "Fullness" and "Aftertaste" within Resource vanilla stimulus. Comparing stimuli, significant differences were found between the attributes "Smell" (p = 0.002),"Sweetness" (p < 0.0005) and in the overall CTS (p = 0.011). The mean scores for each stimulus in the discriminating attributes are presented in table III.
Scaling
The scaling model comparing nutritional supplement grouping versus gazpacho (nominal dependent variable), using attributes as predictors, and response categories as nominal, attained a R2 value of 0.362 (p < 0.001). The model was able to detected significant differences between the two types of stimuli (p < 0.001). Madrid scale significant attributes were: "Appearance", "Smell", "Taste", "Sweetness" and "Opinion", while "Texture" was close to significance, and"Aftertaste" and "Fullness" where not significant. Most discriminating attributes where "Sweetness" and "Smell". Attribute coefficients and discriminating significance are shown in table IV.
The model comparing nutritional supplements was only able to detect differences between different stimuli (R2 = 0.144, p = 0.036). "Appearance", "Texture", "Taste", "Aftertaste" and "Opinion" attributes were significantly sensitive to differences between supplements; while the attribute "Fullness" was close to significance and the attributes "Smell" and "Sweetness" did not show enough discriminating power. Table V shows the stimuli optimum quantifications resulting from the scaling model. The greatest discrimination occurred between Glucerna SR chocolate and Clinutren vanilla, which represent the most and least preferred stimuli, respectively (quantification sign is inversely coded using overall opinion scale as bench mark) (fig. 1).
On the extreme of the positive quantifications (less preference) are the quantifications for Clinutren vanilla and Resource vanilla, which differed only slightly. The quantifications for Glucerna SR chocolate and Glucerna SR strawberry were also very close, but in this case on the extreme of the negative quantifications (great preference). Finally, Diasip vanilla received a quantification similar to that of the anchor stimulus Glucerna SR vanilla, with only a small difference between them. Additionally, when ordinality of the attribute categories is not assumed, the preference ranking of stimuli differs slightly from that obtained using the mean CTS score for each stimulus.
Discussion
The present study was proposed with a dual objective; first, to design and validate, from a classic psychometric perspective, a brief scale for assessing nutritional supplements for diabetic patients, and second, to discover those taste attributes which contribute to discriminate nutritional stimuli and hence rank the different supplements in terms of their acceptance. The Madrid scale has shown to be a valid and reliable instrument which is feasible to be used in daily clinical practice. It also allowed ranking the supplements used in the study according to their preference by type 2 diabetic patients.
No specific guidelines are available for patients with diabetes who are at risk of malnutrition, requiring nutritional support. Most of the modifications in enteral diets specifically for diabetic patients have focused on selecting the type of macronutrient that best controls postprandial glucose excursions, either by combining the percentage of carbohydrates and monounsaturated fat or by adding a different proportion of insoluble or soluble fibre.2 It has thus been found that, compared to standard formulations, these adapted enteral diets achieve better glycemic control both in patients with diabetes3, 23-26 and with stress hyperglycaemia.27 Success of nutritional therapy in malnourished subjects28 is often compromised by the lack of supplements acceptance by the target patients. This represents a double problem in the case of elderly diabetic patients, since both, disease15-19 and age,20 can lead to alterations in the sense of taste, and choices made by the patients' caregivers, physicians, nurses, and dieticians, do not necessarily meet their own preferences.6 In this context, the palatability of the nutritional supplements plays a decisive role in the product acceptance and therefore in its therapeutic compliance.
Among the various studies that have evaluated the preferences of diabetic patients, of particular note is the modification and translation into Spanish of the Wine-Tasting Scale,8 which has been previously used to evaluate nutritional supplements in cancer patients,5 to evaluate drugs29 and, more recently, to evaluate nutritional supplements in diabetic patients.9 However, the psychometric properties of this scale have not been clearly determined. Therefore we proposed to develop a specific questionnaire (the Madrid scale) including all items considered relevant to establish preference for a nutritional supplement, as expressed by specialists and diabetic patients.
With regard to the feasibility of the instrument, the Madrid scale was administered as an interview with the aid of a nurse. Under these conditions, it was completed in less than 5 minutes by all patients, and 100% of the questions were answered on all administrations. Due to the small repertoire of response categories, a ceiling effect was frequently observed. This result cannot be interpreted conclusively because the metric range of the attributes was intentionally small to facilitate assessment, and the stimuli used are very homogeneous and specifically designed to be pleasant to the patients. Nevertheless, the overall score does not present such ceiling effect, although it is negatively skewed, as it is frequent in preference and customer satisfaction scales.
Regarding the dimensionality of the questionnaire, the exploratory factor analysis yielded a one-dimensional solution, which accounts for 45.1% of the total variance. This result is in accordance with the aims of the study and experts assumption. An increase in the attributes metric could possibly convey an increase in the amount of variance explained, but the obtained value has been considered enough to justify the usage of an additive overall score.
Internal consistency and temporal stability are good as shown by a Cronbach's α of 0.809, a test-retest correlation index of 0.717, and a significant intraclass correlation coefficient of 0.835.
Considering different aspects of validity, content validity is supported by construction of the scale on the attributes selected by the panel of experts and ratified by the discussion groups. Construct validity is supported by the factor solution which indicates a structure with a single dimension. Concurrent validity is ensured by the CTS correlation with other scales which are thought to measure the same construct. Significant correlations of 0.774 with the overall score of the Modified Wine-Tasting Scale and of 0.731 with the criterion question where attained. Construct convergent and discriminating validities have been assessed based on item correlation with matching items of the concurrent scale. Correlations of Madrid scale attributes were higher with the equivalent attributes of the Modified Wine-Tasting Scale than with non equivalent ones. All attributes were convergent with their matching attribute and were discriminated from non matching ones, except "Taste" which was found to be interpreted as a transversal quality and "Texture" which did not correlate with most similar attribute "Body". Attribute's scale direction was in agreement with those of the Modified Wine-Tasting Scale and the overall rating item.
Discriminating validity was addressed from two different perspectives, discrimination between subjects with different characteristics versus the same stimulus and discrimination between subjects of the same characteristics versus different stimuli. With regard to subject discrimination, it has been suggested that diabetic patients have altered taste sensations as compared with non-diabetic patients, and sense differences are also expected in elderly versus younger subjects, and smokers versus non-smokers. Only the first two of these three hypotheses could be tested, since the number of smokers in the study sample was very small, and allowed no comparison. Although slow-onset hypogeusia is associated with diabetes,17 our study did not detect differences between controls and diabetics, possibly because of the small number of controls used, although it did find differences between elderly patients in the younger and older age groups.20 The Madrid scale showed a greater capacity of discrimination between stimuli than between subjects. Significant differences were found in the pairwise metric comparison of stimuli for the attributes "Smell" and "Sweetness", and for the CTS.
Due to the number of stimuli involved in the study, It was impossible to present each subject with all pairs of stimuli in a binary comparison (27 = 128 pairs per subject) in order to rank stimuli according to patients' preferences. Therefore it was decided to use an incomplete randomization in which each subject made a limited number of comparisons, although the overall group of subjects included all comparisons of interest, including a very different stimulus, gazpacho, as well as an anchor stimulus, Glucerna SR vanilla.
The problem of preference ordering was addressed in more depth using multidimensional scaling, which does not assume that the attribute measurement is necessarily ordinal. First, the differential salty stimulus (gazpacho) was compared to the supplement grouping (table IV), where it was found that the Madrid scale discriminated between both groups. Differences were found in the attributes "Appearance", "Smell", "Taste", "Sweetness"and "Opinion",while "Texture" was close to significance, and the attributes "Fullness" and "Aftertaste"did not discriminate.
When nutritional supplements were compared to each other (excluding gazpacho), the attributes "Appearance", "Texture", "Taste", "Aftertaste" and "Opinion" were significantly sensitive to the differences between supplements, while the attribute "Fullness" was close to significance. The largest difference was found between the supplements Glucerna SR chocolate and Clinutren vanilla, which are the most and least preferred stimuli, respectively (fig. 1). Based on the plot of the quantifications, the stimuli can be divided into three groups: a high preference group formed by Glucerna SR chocolate and Glucerna SR strawberry, an intermediate preference group formed by the anchor stimulus, Glucerna SR vanilla, and Diasip vanilla, and a low preference group formed by Resource vanilla and Clinutren vanilla. Even if the attribute discriminating capacity has been addressed, this result should be taken with care, since scaling methods tend to assign the 0 reference quantification value to those response categories and objects with more mass (frequency). This is the case of the anchor stimulus which in fact is allocated near the null quantification.
Better acceptance of Glucerna SR® versus Resource diabet® has also been shown recently using a modified wine-tasting scale in a population of 456 patients with type 2 diabetes from nursing homes in Spain.9 Discrimination between the different supplements is substantially lower than when they are compared to gazpacho, due to the similarity in organoleptic characteristics, and large homogeneity of the supplements. As it did not use an ordinal metric, multidimensional scaling found an ordering of the preferences that was slightly different from that obtained with classic test theory. The stimulus with the highest level of preference was the same in both cases, Glucerna SR chocolate, followed by Glucerna SR strawberry. Glucerna SR vanilla was ranked in an intermediate position, both in the classic model and with multidimensional scaling, while Diasip vanilla, which was one of the least preferred stimuli in the classic model, moved to the intermediate group when multidimensional scaling was used. Taking into account that the distortion introduced in the measurement of the attributes by the assumption of ordinality is small and that application of the quantifications obtained from dimensional scaling is otherwise complicated, it is practical to adopt the classic model for everyday use of the Madrid scale.
Besides incomplete randomization, other study limitations that should be noted are: the small number of controls used; the fact that not all commercially available presentations were used; and that it was not possible to study the sensitivity to change of the Madrid scale.
In summary, the Madrid scale is an easy-to use tool which has adequate reliability and validity to evaluate the preferences of diabetic patients for enteral nutritional supplements.
References
1. Solerte SB, Gazarusso C, Schifino N, Locatelli E, Destro T, Ceresini G, Ferrari ER, Fioraventi M. Metabolic Effects of orally amino acid in elderly subjects with poorly controlled type 2 diabetes mellitus. Am J Cardiol 2004; 93:23A-29A. [ Links ]
2. Potter JM. Oral supplements in the elderly. Curr Opin Clin Nutr Metab Care 2001; 4:21-28. [ Links ]
3. Coulston AM. Clinical experience with modified enteral formulas for patients with diabetes. Clin Nutr 1998; 17(Supl. 2):46-56. [ Links ]
4. American Diabetes Association. Nutrition principles and recommendations in diabetes. Diabetes Care 2004; 27(Supl. 1):S36-S46. [ Links ]
5. Brown RO, Schlegel K, Hall NH, Bernard S, Heizer WD. Taste preferences for nutritional supplements: comparison of cancer patients and healthy controls using a wine-tasting scale. JPEN 1986; 10:490-493. [ Links ]
6. Ferzacca S. Lived food and judgments of taste at a time of disease. Med Anthropol 2004; 23:41-67. [ Links ]
7. Amerine MA, Singleton VL. Wine, 2ª; ed. University of California Press, Berkeley, California, 1977: 312. [ Links ]
8. Sánchez Nebra J, Aníbarro García L, Vázquez Vizoso F, Carabelos Acuña P, Cristóbal García F, García Vázquez, Martínez Almeida R, Gutiérrez Solana V, Fernández García L, Luna Ortíz de Zarate B, Echevarri Guerra C. Enteral nutrition and changes in taste in diabetic patients: a doubleblind prospective study [in Spanish]. Nutr Hosp 1993; 8:561-566. [ Links ]
9. Grau T, Almazán Arjona JA, Luna A, Chamorro Quirós J, Lord Rodríguez T, Casimiro C, Grupo Cooperativo Geriátrico. Evaluation of palatability of two special oral diets for institutionalized elderly diabetics, Glucerna SR® vs Resource Diabet® [in Spanish] Nutr Hosp 2004; 19:292-299. [ Links ]
10. Furlong W, Feeny D, Torrance GW, Goldsmith CH, DePauw S, Zhu Z, Denton M, Boyle M. Multiplicative Multi-attribute utility function for the Health Utilities Index Mark 3 System: a technical report, McMaster University Centre for Health Economics and Policy Analysis working Paper 98-11, December 1998. [ Links ]
11. Ruiz MA, Rejas J, Soto J, Pardo A y Rebollo I. Adaptación y validación del Health Utilities Index Mark 3 al castellano y baremos de corrección en la población Española. Med Clin (Barc) 2003; 120:89-96. [ Links ]
12. Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement 1960; 20:141-151. [ Links ]
13. Cattell RB. The Scree test for the number of factors. Multivariate Behavioral Research 1966; 1:245-276. [ Links ]
14. Cattell RB, Vogelmann SA. A comprehensive trial of the scree test and KG criteria for determining the number of factors. Multivariate Behavioral Research 1977; 12:289-325. [ Links ]
15. Le Floch JP, Le Lievre G, Sadoun J, Periemuter L, Peynegre R, Hazard J. Taste impairment and related factors in type I diabetes mellitus. Diabetes Care 1989; 12:173-178. [ Links ]
16. Stolbova K, Hahn A, Benes B, Andel M, Treslova L. Gustometry of diabetes mellitus patients and obese patients. Int Tinnitus J 1999; 5:135-140. [ Links ]
17. Vescovi P, Frigeri S, Caccioli P, Macaluso GM, Oppici A. Dysgeusia in clinical practice. 2. Pathology. Dent Cadmos 1991; 59:68-75. [ Links ]
18. Schiffman SS. Taste and smell in disease. N Engl J Med 1983; 308:1275-1279. [ Links ]
19. Hardy SL, Brennand CP, Wyse BW. Taste thresholds of individuals with diabetes mellitus and of control subjects. J Am Diet Assoc 1981; 79:286-289. [ Links ]
20. Thumfart W, Plattig KH, Schlicht N. Smell and taste thresholds in older people. Z Gerntol 1980; 13:158-188. [ Links ]
21. De Leeuw J, Van Ruckevorsel J. HOMALS and PRINCAL S Some Generalizations of Principal Component Analysis. In: Data analysis and informatics, E. Diday et al., eds. Amsterdam: North-Holland, 1980. [ Links ]
22. Breiman L, Friedman JH. Estimating optimal transformations for multiple regression and correlation. J Am Statistical Assoc 1985; 80:580-598. [ Links ]
23. Coulston AM. Enteral nutrition in the patient with diabetes mellitus. Curr Opin Clin Nutr Metab Care 2000; 3:11-15. [ Links ]
24. Casimiro C, García de Lorenzo A, Usan L and Grupo de Estudio Cooperativo Geriátrico. Nutritional and metabolic status and dietetic evaluation in institutional elderly patients with non-insulin-dependent diabetes mellitus [article in Spanish] Nutr Hosp 2001; 16:104-111. [ Links ]
25. Del Carmen Crespillo M, Olveira G, De Adana MS, Rojo-Martínez G, García-Alemán J, Olvera P, Soriguer F, Muñoz A. Metabolic effects of an enteral nutrition formula for diabetes: comparison with standard formulas in patients with type 1 diabetes. Clin Nutr 2003; 22:483-487. [ Links ]
26. Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specif formulas for patients with diabetes. Diabets Care 2005; 28:2267-2279. [ Links ]
27. Mesejo A, Acosta JA, Ortega C, Vila J, Fernández M, Ferreres J, Sanchos JC, López F. Comparison of a high-protein diseasespecific enteral formula with a high-protein enteral formula in hyperglycemic critically ill. Clin Nutr 2003; 22:295-305. [ Links ]
28. Milne AC, Potter JM, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition (Review). The Cochrane Library 2005; issue 3. [ Links ]
29. Klein K, Lieberman D. A good little antacid. N Engl J Med 1982; 306:1492. [ Links ]
![]() Dirección para correspondencia:
Dirección para correspondencia:
Flor Raigada.
Fundación Gaspar Casal.
C/ General Díaz Porlier, 78-8.º A.
28006 Madrid
E-mail: flor.raigada@fgcasal.org
Recibido: 6-VII-2007.
Aceptado: 6-XI-2007.