Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.25 no.1 Madrid ene./feb. 2010
Parenteral nutrition supplemented with glutamine in patients undergoing bone marrow transplantation
Nutrición parenteral suplementada con glutamina en pacientes sometido a trasplante de médula ósea
L. Alonso Pérez1, A. Fernández Vázquez1, M.a A. Valero Zanuy2, P. Gomis Muñoz1, M. León Sanz2 and A. Herreros de Tejada1
1Pharmacy Service.
2Nutrition Unit. Hospital Universitario 12 de Octubre. Madrid. Spain.
ABSTRACT
The objective of the study is to evaluate if the administration of glutamine in parenteral nutrition (PN) solution reduces the need for antibiotics, the risk of liver disease and the duration of hospital stay in bone marrow transplantation.
Material and methods: Retrospective observational study in 68 adult patients undergoing a bone marrow transplantation who required PN for mucositis. Of these patients, 40 were given PN with 2,063 ± 294 kcal/day and 98.6 ± 13.9 g of amino acids/day, supplemented with Lglutamine (13.5-27 g/day), and 28 were given isocaloric (1,966 ± 307 kcal/day) and isonitrogenated (92 ± 16.3 g of amino acids/day) PN with standard glutamine-free amino acid solution. Antibiotic consumption and duration of hospital stay were analysed. Of the total cohort, hepatic profile was studied at the beginning and on day 7 of PN in 50 patients without liver disease at the start of PN.
Results: There were no differences between both groups with regard to total number and duration of antibiotics prescribed or hospital stay. Of the 50 patients without hepatic alterations at the beginning of PN, 2 patients in the control group and 5 in the glutamine group developed a hepatic profile compatible with liver disease secondary to PN. Comparing both groups, there were no differences in hepatic enzyme values.
Conclusions: Supplementation with PN glutamine does not improve the variables studied, but the actual clinical use of glutamine in this haematological treatment should be studied further and its potential advantages identified.
Key words: Nutrition. Glutamine. Transplantation. Bone marrow.
RESUMEN
El objetivo del estudio es determinar si la administración de glutamina en la solución de nutrición parenteral (NP) disminuye la necesidad de antibióticos, el riesgo de hepatopatía y la duración de la estancia hospitalaria en trasplante de células hematológicas.
Material y método: Estudio observacional retrospectivo, con 68 pacientes adultos sometidos a trasplante de células hematológicas, que precisaron NP por mucositis. De ellos, 40 pacientes recibieron NP con 2.063 ± 294 kcal/día y 98,6 ± 13,9 g de aminoácidos/día, suplementada con L-glutamina (13,5-27 g/día) y 28 recibieron una NP isocalórica (1.966 ± 307 kcal/día) e isonitrogenada (92 ± 16,3 g de aminoácidos/día) con solución de aminoácidos estándar libre de glutamina. Se analizó el consumo de antibióticos y la duración de la estancia hospitalaria. De la cohorte total, en 50 pacientes sin hepatopatía al inicio de la NP se estudió el perfil hepático al inicio y en el día 7 de la NP.
Resultados: No hubo diferencias entre ambos grupos respecto al número total y duración de antibióticos prescritos, ni en estancia hospitalaria. De los 50 pacientes sin alteraciones hepáticas al inicio de la NP, 2 pacientes en el grupo control y 5 en el grupo glutamina desarrollaron un perfil hepático compatible con hepatopatía secundaria a NP. Comparando ambos grupos no hubo diferencias en los valores de enzimas hepáticas.
Conclusiones: La suplementación con glutamina de NP no mejora las variables estudiadas, pero se debe continuar investigando el uso clínico real de glutamina en este tratamiento hematológico, identificando sus potenciales ventajas.
Palabras clave: Nutrición. Glutamina. Trasplante. Médula ósea.
Introduction
Bone marrow transplantation is currently regarded as a potentially curative treatment of different haematological diseases and some solid tumours. Its prognosis has improved in recent years, particularly due to the progress made in the management of infectious complications and in immunosuppression. However, it is not a risk-free therapy. The adverse effects of the different drugs used and total body irradiation, together with the complications specific to the transplantation (graft versus host disease and veno-occlusive disease) are compounded by the lesion on the fast-replication cells. The cells of the immunological system and the gastrointestinal tract belong to this group.1,2 On the one hand, bone marrow aplasia is induced, with the risk of infection. On the other hand, digestive integrity is affected, with alteration of oral intake capacity and nutrient absorption. Both factors may compromise the patient's nutritional status.3,4
Glutamine is the most abundant free amino acid in the human body. Not only does it act as a precursor of protein synthesis, it is also an intermediary in a large number of metabolic pathways. It is a substrate for the renal synthesis of ammonium, and therefore it is involved in the regulation of the acid-base equilibrium. Moreover, it behaves as a source of energy for cells with a high replication rate, such as the intestinal mucosa cells.6-8
Since patients undergoing bone marrow transplantation frequently require artificial nutrition due to the presence of mucositis, the use of glutamine has been tested, either enterally or parenterally, in the treatment of these patients.
The objective of our study was to evaluate whether the administration of glutamine in parenteral nutrition (PN) reduces the need for antibiotic treatment, the risk of liver disease and the hospital stay of patients undergoing bone marrow transplantation.9
Material and methods
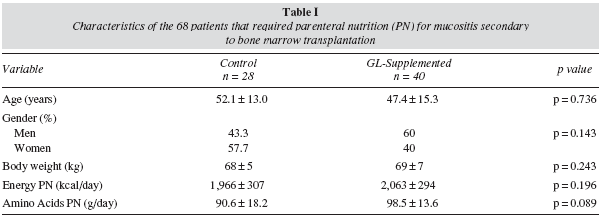
A longitudinal retrospective observational study was performed, including all adults patients undergoing a bone marrow transplantation who required PN for mucositis between January 2006 and January 2008 in our hospital. Of these patients, 28 were given PN with a standard glutamine-free solution of amino acids (control group) and 40 patients were given isocaloric and isonitrogenated PN supplemented with L-glutamine (13.5-27 g/day) (glutamine group). Age, gender, body weight and the composition of the PN were similar in both groups (table I). We analysed consumption of antibiotics per patient and the number of days over which the patients needed treatment with antibiotics, recorded in the data base of the single-dose dispensing programme. Moreover, the presence of liver disease was studied in the first and seventh day of treatment with PN by means of the hepatic profile obtained from fasting peripheral blood. Liver disease secondary to PN was regarded as the presence of hepatic enzymes at least twice the laboratory's reference value, after ruling out another cause of liver disease. Finally, the duration of the hospital stay was also analysed.
Of the 68 patients studied, 50 did not present liver disease on the first day of the treatment with PN: 46% were women and 54% men. 36% (n = 18) of these patients were given a standard solution of amino acids, and 64% (n = 32) received a solution enriched with glutamine dipeptide. The groups were similar in age, gender, body weight and PN composition (table II).
Descriptive statistics, comparison of means for normally distributed quantitative variables and chi square for categorical variables were used for the statistical study. A probability of 95% was regarded as significant.
Results
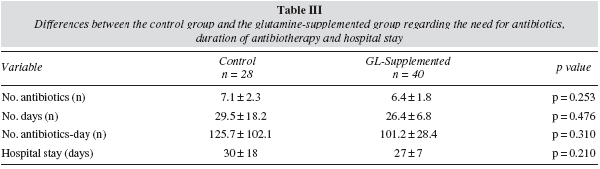
No differences were found between the glutamine group and the control group with regard to total antibiotics prescribed, the number of days with at least one antibiotic or the number of antibiotics-day. The duration of the PN and hospital stay was similar in both groups (table III).
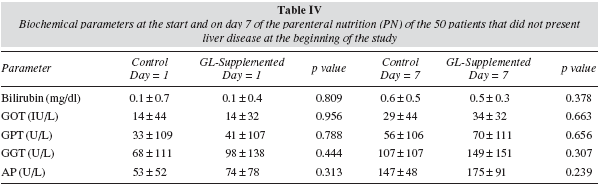
Of the 50 patients that did not present liver disease at the beginning of treatment with PN, 2 patients in the control group (11.1%) and 5 in the glutamine group (15.6%) presented a hepatic profile compatible with liver disease secondary to PN during treatment. GOT, GPT, GGT enzymes and alkaline phosphatase and bilirubin were analysed. Comparing both groups, no significant differences were found in hepatic profile (table IV) or the duration of the PN (9.3 ± 5.1 vs 9.0 ± 3.9 days, respectively).
Discussion
Glutamine is a very abundant amino acid in our body. It has traditionally been regarded as a non-essential amino acid because it can be synthesised from glutamate by the action of glutamate synthetase. It has been confirmed that the needs of this amino acid are augmented in catabolic statuses. A reduction in the levels of plasma glutamine in critical patient has been described. Moreover, the presence of low plasma concentrations correlates inversely with prognosis.10
Glutamine is a fundamental substrate in many metabolic processes, such as the inter-organ transfer of nitrogen, the transport of carbon, the synthesis of nucleic acids and proteins, gluconeogenesis and homeostasis of acid-base equilibrium. Similarly, it is an important source of energy for the intestinal mucosa cells, and other cells with a high rate of replication, such as the immune system cells.11
Traditionally, PN solutions do not contain glutamine. The intravenous administration of amino acids presents two fundamental problems. The first one is its low water solubility, which is only 36 g/l at 20o C. The second one is its low chemical stability in an aqueous solution at 22-24o C. These problems have led to the development of two glutamine dipeptides of greater solubility and chemical stability: L-alanyl-L-glutamine and L-glycyl-L-glutamine. It has been demonstrated that PN solutions supplemented with glutamine improve nitrogen balance12,13 and reduce muscular loss of this substance during stress.14
The administration of glutamine in patients undergoing bone marrow transplantation and receiving high doses of chemotherapy, be it as free amino acid or dipeptide, given enterally or parenterally, has been studied in different works. The studies performed to date have demonstrated, first of all, the safety of parenteral administration, and secondly that it has several beneficial effects versus standard PN solutions. The beneficial effects described include a significant reduction in hospital stay (between 6 to 8 days' difference according to some studies),15 in days with diarrhoeaiii5, in hospital costs,15 in the number of positive blood cultures, 16 the number of infections,16 net urine nitrogen loss over 7 days,16 the urine excretion of 3-methylhistidine, 16 total body water17 and extracellular liquid expansion,16 among others. A significant improvement has also been observed in the circulating lymphocyte recovery time: total circulating lymphocytes, T lymphocytes, helper T cells (CD4) and suppressor T cells (CD8)17 and the score on mood scales.15 Moreover, a metaanalysis was published in 2002 which demonstrated that the administration of glutamine reduces the duration of hospital stay.16 However, another study showed no significant differences in the incidence of positive cultures, in the incidence of infections and mortality between the control group and the glutaminesupplemented group.17 Moreover, Pytlik et al.18 found clearly deleterious effects with the supplementation of PN with glutamine. They observed more severe oral mucositis (p = 0.04), longer duration of the treatment with opioids (p = 0.03), longer duration of hospital stay (p = 0.06), greater mortality (p = 0.05) and greater economic cost (p = 0.002). However, other papers did not confirm a worsening of the incidence and/or the severity of the mucositis.16,17 . The cause of the controversy in the results obtained in the different studies may be due to the heterogeneity of the population studied.15
In our study we found no significant differences in the need for treatment with antibiotics, the number of days over which patients needed treatment with antibiotics, the incidence of liver disease or the duration of the need for PN or the hospital stay of the patients undergoing bone marrow transplantation. A study with a greater number of patients may give a different result.
In conclusion, a retrospective analysis of routine clinical practice finds no additional advantages to the supplementation of PN with glutamine regarding the use of antibiotics, duration of the use of PN and hospital stay, as well as the development of liver disease secondary to PN. The reasons for the discrepancy with other authors may be due to differences in patient types, the doses of glutamine used, as well as the randomisation and intensity of the clinical and analytical monitoring. However, it will be interesting to verify whether future studies on the clinical use of supplementation with glutamine endorse the absence of clinical impact observed in our study.
References
1. Thomas ED, Clift RA, Store R. Indications for bone marrow transplantation. Annu Rev Med 1984; 35-1-9. [ Links ]
2. McDiarmid S. Nutritional support of the patient receiving highdose therapy with hematopoietic stem cell support. Can Oncol Nurs J 2002; 12: 102-15. [ Links ]
3. Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ, Booth IW. Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch Dis Child 1996; 75: 208-213. [ Links ]
4. Klein S, Koretz RL. Nutrition Support in Patients with cancer: What do the data really show? NCP 1994; 9: 91-100. [ Links ]
5. Ziegler TR, Young LS, Benfell K, Scheltinga M, Hortos K, Bye R, Morrow FD, Jacobs DO, Smith RJ, Antin JH et al. Clinical and metabolic efficacy of glutamine- supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med 1992; 116: 821-8. [ Links ]
6. Meister, A. Metabolism of glutamine. Physiol 1956; 36: 103-127. [ Links ]
7. Phromphet-Charat V, Jackson PD, Dass and TC. Welbourne Ammonia partitioning between glutamine and urea: interorgan participation in metabolic acidosis. Kidney Int 1981; 20: 598-605. [ Links ]
8. Sies H, Häussinger D. Hepatic glutamine and ammonia metabolism. Glutamine metabolism in mammalian tissues. D. Häussinger and H. Sies. Berlin, Heidelberg 1984 Springer Velarg: 78-97. [ Links ]
9. Young LS, Bye M, Scheltinga TR, Ziegler DO, Jacobs, Wilmore DW. Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. JPEN 1993; 17: 422-7. [ Links ]
10. Plank LD, Hill GL. Similarity of changes in body composition in intensive care patients following severe sepsis or major blunt injury. Ann N Y Acad Sci 2000; 904: 592-602. [ Links ]
11. Bociek RG, Stewart DA, Armitage JO. Bone marrow transplantation-current-concepts. J Invest Med 1995; 43: 127-35. [ Links ]
12. Sthele P, Zander J, Mertes N et al. Effects of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1989; 1: 231-233. [ Links ]
13. Jian ZM, Cao JD, Zhu XG et al. The impact of alanil-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double- blind controlled study of 120 patients. JPEN 1999; 23: S62-S66. [ Links ]
14. Roth EJ, Karner G, Ollenschlager A, Simmel P, Fust, Funovics. Alanylglutamine reduces muscle loss of alanine and glutamine in post-operative anaesthetized dogs. Clin Sci (Lond) 1988; 75: 641-8. [ Links ]
15. Muscaritoli M, Grieco G, Capria S, Iori AP, Fanelli FP. Nutritional and metabolic support in patients undergoing bone marrow transplantation. Am J Clin Nutr 2002; 75: 183-190. [ Links ]
16. Schloerb PR, Amare M. Total parenteral nutrition with glutamine in bone marrow transplantation and other clinical applications. JPEN 1993; 17: 407-413. [ Links ]
17. Ziegler RR, Lorraine S, Toung RD, Benfell K, Extinga M, Hortos K, Rancy B, Morrow F, Jacobs D, Smith RJ, Wilmore DW. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. Ann Intern Med 1992; 116: 821-828. [ Links ]
18. Pytlik R, Benes P, Patorkova M, Chocenska E, Gregora E, Prockazka B. Standardised parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: a randomized, doublé-blind, placebo controlled study. Bone Marrow Transplant 2002; 30: 953-961. [ Links ]
![]() Correspondence:
Correspondence:
Lucía Alonso Pérez.
Servicio de Farmacia.
Hospital 12 de Octubre.
Avd. Córdoba, s/n.
28041 Madrid.
E-mail: luci8216@hotmail.com
Recibido: 6-VII-2009.
Aceptado: 6-VII-2009.