Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.4 Madrid jul./ago. 2011
Double blind randomized clinical trial controlled by placebo with an alpha linoleic acid and prebiotic enriched cookie on risk cardiovascular factor in obese patients
Ensayo clínico aletorizado doble ciego controlado con placebo con una galleta enriquecida en ácido alfa linoleico y prebióticos en el patrón de riesgo cardiovascular de pacientes obesos
D. A. de Luis1, B. de la Fuente1, O. Izaola1, R. Conde1, S. Gutiérrez2, M.a Morillo3 and C. Teba Torres3
1Instituto de Endocrinología y Nutrición. Facultad de Medicina y Unidad de Apoyo a la Investigación. Hospital Río Hortega. Universidad de Valladolid.
2División de Investigación Gullon, S. A.
3División de Alimentación. Centro de Automatización Robótica y Tecnologías de la Información y la Fabricación CARTIF. Spain.
ABSTRACT
Introduction: Inulin and FOS are prebiotics with potential benefit in cardiovascular risk factors. Alpha linolenic acid (ALA) is the metabolic precursor of the long chain n-3 fatty acid eicosapentaenoic acid (20: 5n-3), this fatty acid has anti-inflammatory properties. The aim of our study was to evaluate the response of the cardiovascular risk profile in obese patients after inclusion in the diet of an ALA, FOS and inulin enriched-cookie.
Material and methods: 36 patients were randomized in both branches: group I (inulin, FOS and ALA enriched cookie) Gullon SL® and group II (control cookie). Previous and after 1 month of the treatment, a nutritional and biochemical study was realized.
Results: 15 patients finished the procotol in each group. In group I, a significantly increase in soluble fiber (2.3 ± 0.8 g/day vs 7.7 ± 0.8 g/day: p < 0.05) and ALA (0.6 ± 0.5 g/day vs 3.8 ± 0.5 g/day; p < 0.05) intakes was detected. In this group a significant decrease of total cholesterol (238.1 ± 45.3 mg/dl vs 210.5 ± 38.1 mg/dl: p < 0.05), LDL cholesterol (153.6 ± 23.2 mg/dl vs 127.1 ± 27.9 mg/dl: p < 0.05) and C reactive protein (6.6 ± 1.4 mg/dl vs 4.4+7-1.8 mg/dl: p < 0.05) was reached in males. Anthropometric parameters did not change in both groups. The increase in soluble fiber and ALA dietary intakes did not produce any gastrointestinal adverse effect.
Conclusion: The increase of 2 grams per day of inulin, 3.1 grams per day of FOS and 3.2 grams per day of alpha linolenic (ALA) dietary intakes from an enriched-cookie, improved total cholesterol, LDL cholesterol and C reactive protein levels in obese males. As far as we know, this is the first study that has evaluated the effect on risk factors of an ALA enriched cookies.
Key words: Acid alpha linolenic. Cardiovascular risk factors. Cookies. FOS. Inulin. Obesity.
RESUMEN
Introducción: La inulina y los FOS son prebióticos con potenciales efectos beneficiosos a nivel cardiovascular. El acido alfa linolénico (ALA) es el precursor del ácido eicosapentaenoico (20: 5n-3), presentando propiedades antinflamatorias. El objetivo de nuestro trabajo es evaluar la respuesta del perfil de riesgo cardiovascular en pacientes obesos tras la inclusión en la dieta de una galleta enriquecida en inulina, FOS y ALA.
Material y métodos: Un total de 36 pacientes fueron randomizados a una de las siguientes ramas: galleta I (enriquecida con inulina, FOS y ALA) y galleta II (galleta control) (Gullón SL). Cada paciente recibió un total de 2 galletas al día (70 gramos de producto). Antes de iniciar el tratamiento y al mes, se realizó una valoración nutricional y analítica.
Resultados: Finalizaron el protocolo un total de 15 pacientes en cada grupo. En el grupo I se produjo un aumento significativo en la ingesta de fibra soluble (2,3 ± 0,8 g/día vs 7,7 ± 0,8 g/día: p < 0,05) (inulina y FOS), así como ALA (0,6 ± 0,5 g/día vs 3,8 ± 0,5 g/día; p < 0,05). Se detectó en los pacientes varones que recibieron las galletas enriquecidas una disminución significativa de los niveles de colesterol total (238,1 ± 45,3 mg/dl vs 210,5 ± 38,1 mg/dl: p < 0,05), LDL colesterol (153,6 ± 23,2 mg/dl vs 127,1 ± 27,9 mg/dl: p < 0,05) y proteína C reactiva (6,6 ± 1,4 mg/dl vs 4,4+7-1,8 mg/dl: p < 0,05). No existieron diferencias estadísticamente significativas en las variables antropométricas. El aumento de la ingesta dietética de fibra soluble y ALA en los pacientes de grupo I no supuso ningún efecto secundario a nivel gastrointestinal.
Conclusión: El aumento en la ingesta con una galleta enriquecida de 2 gramos al día de inulina, 3,1 gramos de FOS y 3,2 gramos de ALA, mejora en los pacientes obesos varones los niveles de colesterol total, LDL colesterol y proteína C reactiva.
Palabras clave: Ácido alfa linolénico. Factores de riesgo cardiovascular. Galletas. FOS. Inulina. Obesidad.
Introduction
Obesity now represents a major pandemic, with a multifactorial origin, showing an association with various cardiovascular risk actors, high mortality and high healthcare costs. In our country the prevalence of obesity is at 13%, and overweight over 30%.1 Therapeutic options for the treatment of obesity go through dietary management,2 drug therapy and bariatric surgery.3 Despite the wide range of treatments, the first therapeutic step is the dietary treatment. Diet has proven effective in weight loss and improvement in cardiovascular risk parameters. One of the problems of dietetic therapy is the lack of patient adherence, and lack of perception of the benefits secondary to the control of cardiovascular risk factors. One possibility is included in the diet, heart-healthy foods that include changes in composition as fiber, fats or vitamins. Cookies are one of the foods that has been modified to improve this cardiovascular risk. Several studies have demonstrated the usefulness of these foods, eg Romero et al.4 have proven useful in lowering cholesterol psyllium-enriched cookies. Other groups have shown improvement in cardiovascular risk factors with the use of bread or cookies enriched in beta-glucans.5-6 Inulin, which include fructooligosaccharides (FOS), oligofructose and inulin, are the most common prebiotics commercially and those with a greater number of studies that have examined their actions on health and may present a potential role in controlling certain cardiovascular risk factors for obese patients.7
Other healthy nutrients are poly-unsaturated fatty acids. For example, alpha linoleic acid (ALA) has cardiovascular propierties, too. The cardioprotective mechanisms of ALA may include the prevention of ventricular fibrillation,8 decrease response to aggregation.9 Furhtermore, ALA is the metabolic precursor of the long chain n-3 fatty acid eicosapentaenoic acid (20: 5n-3), this fatty acid has anti-inflammatory properties.
The aim of our study was to evaluate the response of the cardiovascular risk profile in obese patients after inclusion in the diet of an ALA and prebiotic enrichedcookie. As far as we know, this is the first study that has evaluated the effect on risk factors of an ALA and prebiotic enriched cookies.
Material and methods
The sample consisted of 36 obese patients (BMI >30), starting the recruitment in august 2009 and completed follow-up of patients in july 2010. These patients were studied in a Nutrition Unit, all patients signed an informed consent protocol. The protocol has been approved by the Ethics Committee of the Center. Exclusion criteria were: elevated blood glucose > 126 mg/dl, high cholesterol > 250 mg/dl, triglycerides > 250 mg/dl, blood pressure > 140/90 mmHg, and the taking of any of the following medications; statins, fibrates, resins, sulfonylureas, biguanides, thiazolidinediones, insulin, glucocorticoids, alpha blockers, converting enzyme inhibitors and angiotensin II receptor antagonists, angiotensin.
Procedure
Patients were randomized (table of numbers) to one of the following two groups: cookie I (enriched with inulin, FOS and ALA, see table I) (Gullón SL) and cookie II (control cookie, see table I). Each patient received a total of 2 cookies per day (total product 70 grams), completing a month of treatment. Cookie intake was controlled for a month. The methodology was double-blind, neither the patient nor the investigator who followed the patient knew the type of cookie.
Before starting the dietary intervention and at the end of the protocol were determined dietary intake, weight, fat mass, blood pressure, fasting blood glucose, C reactive protein (CRP), insulin, insulin resistance (HOMA), total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides.
Biochemical determinations
Serum total cholesterol and triglyceride concentrations were determined by enzymatic colorimetric assay (Technicon Instruments, Ltd., New York, N.Y., USA), while HDL cholesterol was determined enzymatically in the supernatant after precipitation of other lipoproteins with dextran sulphate-magnesium. LDL-cholesterol was calculated using Friedewald formula. Plasma glucose levels were determined by using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, California). Insulin was measured by enzymatic colorimetry (Insulin, WAKO Pure-Chemical Industries, Osaka, Japan) and the homeostasis model assessment for insulin resistance (HOMA)was calculated.10
Anthropometric measurements
Body weight was measured to an accuracy of 0.1 kg and body mass index computed as body weight/ (height2) (kg/m2). Waist (narrowest diameter between xiphoid process and iliac crest) and hip (widest diameter over greater trochanters) circumferences to calculate waist-to hip ratio (WHR) were measured, too. Tetrapolar body electrical bioimpedance was used to determine body composition.11 An electric current of 0.8 mA and 50 kHz was produced by a calibrated signal generator (Biodynamics Model 310e, Seattle, WA, USA) and applied to the skin using adhesive electrodes placed on right-side limbs. Resistance and reactance were used to calculate total body water, fat and fat-free mass.
Blood pressure was measured twice after a rest period of 10 minutes with a random zero mercury sphygmomanometer (Omron, London, United Kingdom), and averaged.
Dietary intervention
Before and after intervention, patients received prospective serial assessment of nutritional intake with 3 days written food records. All enrolled subjects received instruction to record their daily dietary intake for three days including a weekend day. Handling of the dietary data was by means of a personal computer equipped with personal software, incorporating use of food scales and models to enhance portion size accuracy. Records of intake and consumption of cookies were reviewed by a dietician and analyzed with a computer- based data evaluation system. National composition food tables were used as reference.12 The exercise allowed was aerobic, which was previously done by patients before entering the study, mainly walking. At dietary intervention, patients were asked whether they considered their bowel habits have changed over who had previously shown a quantitatively and qualitatively. For a qualitative evaluation, they were asked whether they considered that the introduction of the cookie in the diet would have produced diarrhea or constipation. For the quantitative point of view they were asked the number of stools per week during the month preceding the intervention and during the intervention month.
Statistical analysis
The sample size was calculated to detect a difference in C reactive protein levels after treatment of 1 mg/dl with a 90% power and an alpha error of 5% (n = 15 in each group). The results were expressed as mean (standard deviation). The normality of variables was analyzed by the Kolmogorov-Smirnov. Quantitative variables with normal distribution were analyzed with Student's t test paired and unpaired. Variables without normal distribution were analyzed with Wilcoxon Wtest. Qualitative variables were analyzed with chisquare with Yates correction when appropriate, and Fisher's test. The strategy of analysis was by intention to treat. P less than 0.05 was considered statistically significant.
Results
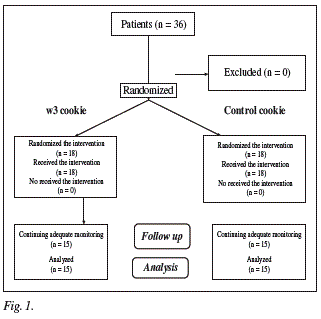
36 patients were included in the protocol (fig. 1, Consort diagram), 30 patients finished the study. The 6 patients excluded from the analysis had taken less than 80% of the prescribed cookies. The distribution was in group 1 (6 males and 9 females) with a mean age of 50.6 ± 15.2 years and the control group 2 (6 males and 9 females) with a mean age of 50.8 ± 15.1 years. No differences in gender and age distribution of patients were observed. Baseline values of anthropometric and biochemical parameters were similar in both groups (table II).
With respect to the anthropometric parameters after the introduction of cookies on the patient's usual diet, did not change any parameter (table II). This finding is logical because the inclusion of patients in the protocol did not alter total energy intake from their diet. With respect to the biochemical values after the introduction of cookies on the patient's usual diet, it was detected in patients with enriched cookies a trend to significantly reduced levels of LDL cholesterol (p = 0,078) and C reactive protein (p = 0,092) (table II).
Table III shows the anthropometric and biochemical parameters in males (n = 12). After treatment, no differences were detected in anthropometric parameters. Total cholesterol (238,1 ± 45,3 mg/dl vs 210,5 ± 38,1 mg/dl: p < 0,05), LDL cholesterol (153,6 ± 23,2 mg/dl vs 127,1 ± 27,9 mg/dl: p < 0,05) and C reactive protein (6,6 ± 1,4 mg/dl vs 4,4+7-1,8 mg/dl: p < 0,05) decreased significantly in group I (enriched cookie).
Table IV shows the anthropometric and biochemical parameters in females (n = 18). After treatment, no differences were detected in anthropometric and biochemical parameters.
In the evaluation of dietary intake variables, no statistically significant differences between baseline values of the two groups of cookies were detected (table V). With respect to the values after the introduction of cookies on the patient's usual diet, it was detected in patients with ALA enriched cookies a significantly increased of total fiber, soluble fiber and ALA dietary intakes. It was not detected any significant change in food intake in patients who received the control cookie (table V). No differences between soluble fiber and ALA intakes were detected between males and females (data not shown).
The number of cookies given per patient per month was 60 cookies. The number of consumed cookies after a month of intervention was 53,87 ± 3,4 (89,7%) in patients in the control cookie and 53,73 ± 4,8 in patients in the cookie enriched (89,6%), without statistical differences. We could summarize the improvement in biochemical parameters in terms of one gram of soluble fiber intake increased with enriched cookie according to the following direct relationships; a decrease of total cholesterol 6,22 ± 3,28 mg/dl, LDL cholesterol 5,70 ± 3,20 mg/dl and C reactive protein 2,45 ± 0,93 mg/dl. In terms of one gram of ALA intake increased with enriched cookie, we detected a decrease of total cholesterol 9,1 ± 8,3 mg/dl, LDL cholesterol 8,44 ± 7,27 mg/dl and C reactive protein 0,69 ± 0,73 mg/dl.
With respect to monitoring the effects on the digestive tract, one patient in the group of control cookies (6,7%) referred episodes of diarrhea during the month of treatment. Two patients (13,4%) in the control group and 1 patients in the enriched cookie group (6,7%) referred have constipation during the month of intervention. However, comparing the average weekly number of stools the month preceding the study and the month during the study, no statistically significant differences were observed; enriched cookie (8,6 ± 4,4 stools/week vs 9,6 ± 5,3 stools/week) and control cookie (8,1 ± 3,9 stools/week vs 8,5 ± 2,4 stools/week).
Discussion
Our work has shown how the inclusion in the diet of a prebiotic and ALA enriched cookie, providing about 2 grams per day of inulin, 3,1 grams per day of FOS and 3,2 grams per day of alpha linolenic (ALA), produced a significant decrease on levels of total cholesterol, LDL-cholesterol and C reactive protein in obese males.
If we analyze the literature we found a number of problems in analyzing our results and the studies previously performed. For example, we could mention, the heterogeneity of the populations (obese, diabetic, hyperlipidemic, healthy subjects, gender of the sample), secondly the daily amount of fiber administered and the type of prebiotic, which can vary from pure inulin to fructooligosaccharides (FOS), thirdly the variability in the time of intervention performed and fourthly the addition of other healthy nutrients such as ALA. For example, one of the earliest studies was conducted with 12 healthy men, found no effect on the lipid profile by adding to the daily diet of 20 g FOS.12 Similarly, in a study with 12 healthy volunteers also in various stages of intervention with inulin, FOS and galacto-oligosaccharides (GOS), there were no effects on total cholesterol, LDL cholesterol, apolipoprotein A-1 and B, triglycerides, HDL cholesterol.13 However, the results were significant when inulin was used in the interventions. Thus, in the study of Letexier et al.,14 administration of 10 g inulin per day versus placebo, showed a significant decrease of triglyceride levels in healthy volunteers. In a randomized clinical trial controlled with placebo,15 after administration of 7 g inulin per day for 4 weeks produced a significant decrease in triglycerides, total cholesterol and LDL cholesterol. In other randomized clinical trial,16 the increase of fiber intake (3 g of inulin) from an enriched cookie reduced LDL cholesterol levels in obese patients. So, we could summarize this group of studies, noting that in the literature beneficial effects on triglycerides and cholesterol LDL by administering inulin have been detected. Most of this effect may be due to increased loss of bile salts in the feces, which can range between 20 and 80%, producing secondarily a decrease in total body cholesterol.17 Another factor involved is the decrease in glycemic response and insulin secretion after administration of this type of soluble fiber.18 Inulin levels are associated with activation of the enzyme hydroxymethyl-glutaryl-coenzyme A reductase, the rate limiting step in cholesterol synthesis. Finally, the bacterial fermentation of this fiber increases the production of short chain fatty acids (SCFA). One of these fatty acids, propionate, can acutely inhibit the cholesterolinduced increase in acetate.18
ALA could play a role in the cardiovascular benefits of our clinical trial, too. ALA is a plant w-3 fatty acid, precursor of docosahexaenoic acid and eicosapentaenoic acid, the two main w3 polyunsaturated fatty acids found in fish. In some studies,19-20 the following markers of inflammation improved; tumor necrosisfactor alpha, interleukin 6, C reactive protein, cell adhesion molecule 1 and vascular cell adhesion molecule. In other interventional study with Salba (Salvia hispanica L.), a novel whole grain that is rich in fiber and ALA, decreased systolic blood pressure and CRP.21 In two randomized controlled trial22-23 conducted in hypercholesteronemica subjects, consumption of ALA diets significantly decreased serum levels CRP. In a systematic review, Wendland et al.24 have shown that ALA supplementation may cause decreases in inflammatory markers (fibrinogen concentrations) and in fasting plasma glucose. The average reduction of fibrinogen levels were 0,17 umol/l attributable to ALA, this small reduction lead to a reduction of 6% in coronary heart disease. This is a smaller reduction than that observed in the Lyon diet heart study,25 in which patients were randomly assigned to a Mediterranean diet and margarine high in ALA (4,8%). ALA is a metabolic precursor of DHA and EPA and any inflammatory improvement may be mediated through conversion to this fatty acids. However, the metabolic overall conversion rate is low and varies between the sexes,26 this fact could explain the different sex metabolic response observed in our study. Moreover, ALA can improve LDL cholesterol, too. For example, in a study of dietary advice with at least 3-4 servings per day of mustard oil or soybean oil (rich in ALA) showed an improvement in LDL cholesterol.27
Our study has some limitations. First, nutrients intakes were derived from a questionnaire. Second, we were not able to separate soluble fiber and alpha linolenic acid effect on metabolic parameters. However, the evaluation of this type of nutritional intervention with fortified foods is of great interest to reduce cardiovascular risk factors present in the obese population. Especially rich in fiber and poly-unsaturated fatty acids foods may have multiple beneficial effects.28-31
In conclusion, the increase in dietary intake of 2 grams per day of inulin, 3,1 grams per day of FOS and 3,2 grams per day of alpha linolenic (ALA) from an enriched-cookie, improved total cholesterol, LDL cholesterol and CRP levels in obese males.
References
1. Aranceta-Bartrina J, Serra-Majem L, Foz-Sala M, Moreno-Esteban B; Grupo Colaborativo SEEDO. Prevalence of obesity in Spain. Med Clin (Barc) 2005; 125 (12): 460-6. [ Links ]
2. De Luis DA, Aller R, González Sagrado M, Izaola O, Conde R. The effects of a low fat versus a low carbohydrate diet on adipocytokines in obese adults. Hormone Research 2007; 67: 296-300. [ Links ]
3. De Luis DA, Pacheco D, Izaola O, Teroba M, Cuellar L, Martin T. Clinical Results and nutritional consequences of biliopancreatic diversion: Three years of follow up. Ann Nutr Metab 2008; 53: 234-238. [ Links ]
4. Romero AL, Romero JE. Cookies enriched with psyllium or oat bran lower plasma LDL cholesterol in normal and hypercholes-teronemic men from northern mexico. J of American College of Nutrition 1998; 6: 601-608. [ Links ]
5. Kerkhoffs D, Hornstra G. Cholesterol-lowering effect of beta glucan from oat bran in mildly hypercholeteronemic subjects may decrease when beta glucan s incorporated into bread and cookies. Am J Clin Nutr2003; 78: 221-227. [ Links ]
6. Keogh G, Cooper G, Mulvey T. Randomized controlled crossover study of the effect of a highly beta glucan enriched barley on cardiovascular disease risk factors in moldly hypercholesterolemic men. Am J Clin Nutr 2003; 78: 711-718. [ Links ]
7. Roberfroid M. Prebiotics: the concept revisited. 1. J Nutr 2007; 137: 830S-837S. [ Links ]
8. Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double blind, placebo controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarctation: the indian experiment of infarct survival 4. Cardiovascular Drugs Ther 1997: 11: 485-491. [ Links ]
9. Freese R, Mutanen M, Valsta SM, Salminen I. Comparison of the effects of two diets rich in monounsaturated fatty acid differing in their linoleic/alpha-linolenic acid ratio on patelet aggregation. Thromb Haemost 1994: 71: 73-77. [ Links ]
10. Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher Df. Homesotasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412-414. [ Links ]
11. Lukaski H, Johson PE. Assessment of fat-free mass using bio-electrical impedance measurements of the human body. Am J Clin Nutr 1985; 41: 810-7. [ Links ]
12. Mataix J, Mañas M. Tablas de composición de alimentos españoles. Ed: University of Granada, 2003. [ Links ]
13. Van Dokkum W, Wezendonk B, Srikumar TS, Van den Heuvel EG. Effect of nondigestible oligosaccharides on large-bowel functions, blood lipid concentrations and glucose absorption in young healthy male subjects. Eur J Clin Nutr 1999; 53: 1-7. [ Links ]
14. Letexier D, Diraison F, Beylot M. Addition of inulin 19. To a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am J Clin Nutr 2003; 77: 559-564. [ Links ]
15. Balcazar-Munoz BR, Martinez-Abundis E, Gonzalez-Ortiz M. Effect of oral inulin administration on lipid profile and insulin sensitivity in subjects with obesity and dyslipidemia. Rev Med Chil 2003; 131: 597-604. [ Links ]
16. De Luis DA, De la Fuente B, Izaola O, Conde R, Gutiérrez S, Morillo M, Teba Torres C. Ensayo clínico aleatorizado con una galleta enriquecida en insulina en el patrón de riesgo cardiovascular de pacientes obesos. Nutr Hosp 2010; 25: 53-59. [ Links ]
17. Malkki Y. Oat fiber. In Handbook of dietary fiber. Edited bu: Cha SS, Dreher ML, New Cork Macrel Dekker 2001; 497-512. [ Links ]
18. Wolever TMS, Brighenti F, Jenkins DJA. Serum short Chain fatty acids alter rectal infusion of acetate and propionate in man. J Clin Nutr Gastroenterol 1988; 3: 42-46. [ Links ]
19. Rallidis LS, Paschos G, Liakos GK. Dietary alpha linolenic acid decreases c reactive protein, serum amyloid A and interleukin in dyslipemic patients. Atherosclerosis 2003; 167: 237-242. [ Links ]
20. Thies F, Miles EA, Nebe van Caron G. Influence of dietary supplementation with long chain n3 or n6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 2001; 36: 1183-1193. [ Links ]
21. Vuksan V, Whitham D, Sievenpiper J, Jenkins A, Rogovik A, Bazinet R, Vidgen E, Hanna A. Supplementation of conventional therapy with the novel grain salba (Salvia hispanica L.) Improves major and emerging cardiovascular risk factors in type 2 diabetes. Diabetes Acre 2007; 30: 2804-2810. [ Links ]
22. Bemelmand WJ, Lefrandt JD, Feskens EJ, Van Haeslt PL, Broer J, Meyboom de Jong B, May JF, Tervaert JW. Increased alpha linolenic acid intake lowers C reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr 2004; 58: 1083-1089. [ Links ]
23. Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense LDL, remmant lipoprotein particles, and c reactive protein in metabolic syndrome. Diabetes Care 2007; 30: 144-146. [ Links ]
24. Wendland E, Farmer A, Glasziou P, Neil A. Effect of alpha linolenic acid on cardiovascular risk markers:a systematic review. Heart 2006; 92: 166-169. [ Links ]
25. De Lorgeril M, Salen P, Martin JL. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarctation: final report of the Lyon diet heart study. Circulation 1999; 99: 779-785. [ Links ]
26. Burdge GC, Woolton SA. Conversion of alpha linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 2002; 88: 411-420. [ Links ]
27. Singh RB, Dubnov G, Niaz MA. Effect of an Indo-mediterranean diet on progression of coronary artery disease in high risk patients (INDO Mediterranean Diet heart Study): a randomized single blind trial. Lancet 2002; 360: 1455-1461. [ Links ]
28. López Román J, Martínez Gonzálvez AB, Luque A, Pons Miñano JA, Vargas Acosta A, Iglesias JR, Hernández M, Villegas JA. The effect of a fibre enriched dietary milk product in chronic primary idiopatic constipation. Nutr Hosp 2008; 23: 12-9. [ Links ]
29. García Peris P, Velasco Gimeno C. Evolution in the knowledge on fiber. Nutr Hosp 2007; 22 (Suppl. 2): 20-5. [ Links ]
30. Lecumberri E, Mateos R, Ramos S, Alía M, Rúperez P, Goya L, Izquierdo-Pulido M, Bravo L. Characterization of cocoa fiber and its effect on the antioxidant capacity of serum in rats. Nutr Hosp 2006; 21: 622-625. [ Links ]
31. Martín de Santa Olalla L, Sánchez Muniz FJ, Vaquero MP. N-3 fatty acids in glucose metabolism and insulin sensitivity. Nutr Hosp 2009; 24: 113-27. [ Links ]
![]() Correspondence:
Correspondence:
D. A. de Luis.
Profesor Asociado de Nutrición.
Director del Instituto de Endocrinología y Nutrición.
Facultad de Medicina. Universidad de Valladolid.
C/ Los Perales, 16. 47130 Simancas. Valladolid. Spain.
E-mail: dadluis@yahoo.es
Recibido: 18-XI-2010.
Aceptado: 28-XI-2010.