Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.26 no.5 Madrid sep./oct. 2011
Influence of fat intake on body composition, lipemia and glycemia of type 1 diabetics
Influencia de la ingesta de grasas en la composición corporal, respuesta inflamatoria y metabolismo de los lipidios de la glucosa en los diabéticos tipo 1
P. Mansur Leal, D. Lopes Souto, E. dos Santos Lima, M. Paes de Miranda and E. Lopes Rosado
Instituto de Nutrição Josué de Castro. Universidade Federal do Rio de Janeiro. Brazil.
ABSTRACT
Background: Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia and body composition is important in the disease control. The nutritional intervention has relevance in the improvement of glycemia and lipemia in diabetic patients.
Aim: Evaluate the influence of fat intake on body composition, lipemia and glycemia on patients with type 1 diabetes mellitus.
Methods: 19 patients were evaluated by anthropometric (body mass index and waist circumference), body composition (fat mass, lean body mass and total body water by bioelectrical impedance) and biochemical variables, after 8 hours of fasting. Dietary assessment was performed using the dietary records for 3 days, analyzed for nutritional software DietPró 5i. The groups were formed according to the usual intake of saturated fatty acids (SFA) (G1 < 10% of total energy expenditure (TEE) of SFA and G2 ≥ 10% of TEE of SFA). Statistical analysis was performed in SPSS 16.0, considering p < 0.05.
Results: There was no difference in anthropometric and biochemical variables between groups, but G1 presented higher fat mass (FM) and G2 high SFA and adequate mounsaturated fatty acids (MUFA) intake. The lipemia and glycemia were not affected by high SFA intake, but adequate MUFA intake may have influenced the results of these variables. No found relation between type of fat ingested and biochemistry variables.
Conclusion: Body composition can be influenced by type of fat ingested. Lipemia and glycemia were not influenced by high SFA intake, perhaps due to MUFA intake adequate.
Key words: Diabetes mellitus. Lipemia. Glycemia. Body composition. Fat intake.
RESUMEN
Introducción: La diabetes mellitus es una enfermedad metabólica caracterizada por hiperglucemia crónica y la composición corporal es importante en el control de la enfermedad. La intervención nutricional tiene relevancia en la mejora de la glucemia y lipemia en pacientes diabéticos.
Objetivo: Evaluar la influencia de la ingesta de grasa en la composición corporal, lipemia y glucemia en pacientes con diabetes mellitus tipo 1.
Métodos: 19 pacientes fueron evaluados por parámetros antropométricos (índice de masa corporal y circunferencia de la cintura), composición corporal (masa grasa, masa corporal magra y agua corporal total por impedancia bioeléctrica) y bioquímicos, después de 8 horas de ayuno. La evaluación dietética se realizó mediante registros dietéticos de 3 días, analizados en el software nutricional DietPró 5i. Los grupos se formaron según la ingesta habitual de ácidos grasos saturados (AGS) (G1 < 10% del gasto energético total (GET) de AGS y G2 ≥ 10% del GET de AGS). El análisis estadístico se realizó en SPSS 16.0, con p < 0,05.
Resultados: No hubo diferencia en los parámetros antropométricos y bioquímicos entre los grupos, pero G1 presentó mayor masa grasa (MG) y G2 mayor ingesta de AGS y adecuada de ácidos grasos monoinsaturados (AGMI). La lipemia y glucemia no fueron afectadas por la elevada ingesta de AGS, pero la ingesta adecuada de AGMI puede influenciar en los resultados de estos parámetros. No fueron verificadas relaciones entre el tipo de grasa y los parámetros bioquímicos.
Conclusión: La composición corporal puede ser influenciada por el tipo de grasa ingerida. La lipemia y la glucemia no fueron influenciadas por la alta ingesta de AGS, tal vez debido a la adecuada ingesta de AGMI.
Palabras clave: Diabetes mellitus. Lipemia. Glucemia. Composición corporal. Ingesta de grasas.
Abbreviations
BMI: Body mass index.
CHO: Carbohydrate.
CVD: Cardiovascular disease.
DM: Diabetes mellitus.
FM: Fat mass.
HbA1c: Glycated hemoglobin.
HDL: High-density lipoprotein.
LBM: Lean body mass.
LDL: Low-density lipoprotein.
MUFA: Monounsaturated fatty acids.
PTN: Protein.
PUFA: Polyunsaturated fatty acids.
SFA: Saturated fatty acids.
TBW: Total body water.
TEE: Total energy expenditure.
TG: Triglycerides.
VLDL: Very-low-density lipoprotein.
WC: Waist circumference.
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycemia resulting from inability to produce and/or secrete insulin.1
The prevalence of DM increases every year. According to the World Health Organization (2003), the number of patients around the world was 177 million in 2000, and expects to reach 350 million in 2025.2
Chronic hyperglycemia cause lower-limb amputations, blindness, chronic kidney disease, risk of developing cardiovascular disease (CVD) is 2-4 times higher and stroke.3 Study with patients with type 1 DM and type 2 DM showed for every 1% reduction in glycated hemoglobin (HbA1c) concentrations decrease in 37% the risk of the complications on DM.4 HbA1c concentrations above 8% indicate the average glucose have been above 200 mg/dL in 3 last months.5
An unfavorable lipid profile may facilitate the foam cells formation in arterial wall and, as triglycerides (TG) concentrations rise, reduced the low-density lipoprotein (LDL) particles become more susceptible to oxidation, a process that further enhances the development of atherogenic lesion.6
Anthropometric measures are important to assess the nutritional status, as help to monitor the possible changes in body composition and choice the most appropriate dietary treatment.7 The body composition, particularly fat mass (FM) and body fat distribution, may contribute to changes in insulin action. The visceral fat accumulation is positively related to high doses of exogenous insulin in type 1 DM.8-9
Type 1 DM treatment must be individualized and involves insulin, glucometer, diet, physical activity, diabetes education and emotional support. The individualized diet plan aims at better glycemic control, reducing the complications associated with the hyperglycemia, lipemia and weight control.2 Inadequate diet is associated with DM uncontrolled.10
The diet plan composition for diabetic patients are similar to recommended for healthy individuals, with 50 to 60% of total energy expenditure (TEE) of carbohydrate (CHO) (15 g of fiber each 1,000 kcal), 25 to 35% of fats (≤10% saturated fatty acids (SFA), < 10% of polyunsaturated fatty acids (PUFA), 10 to 15% monounsaturated fatty acids (MUFA) and ≤ 200 mg/day by cholesterol) and 0,8 to 1g of protein/kg of body weight.1
The high SFA intake is an important determinated factor of the increasing of mortality by CVD, and the American Diabetes Association (ADA) recomends the sequence of control of dyslipidemia in this order, LDL, high-density lipoprotein (HDL) and TG.1
Lipemia, blood glucose, weight and body composition control are important in the prognosis of patients with type 1 DM. Our aim was to evaluate the influence of type of fat intake in these variables in individuals with type 1 DM.
Methods
Sample
A cross-sectional study was carried with 19 patients with type 1 DM, selected on Hospital Universitário Clementino Fraga Filho (6 female (31,57%) and 13 male (63,15%), aged 21,0 ± 2,0 years). We excluded of study volunteers with BMI ≥ 30 kg/m (WHO, 1995) or BMI-for-age > Z-scores + 2, smokers, alcoholic, in use of lipid-lowering or hypoglycemic drug, changes in diet along 3 months or other diseases associated with the DM. The sample was selected for convenience, and reason from fact, the results will be described without the intention of making inferences to other populations.11
The study was approved by the Research Ethics Committee of Hospital Universitario Clementino Fraga Filho (no. 050/09).
Usual dietetic intake was evaluated during three days. Anthropometric, body composition and biochemical variables were assessed, in fasting. Groups formed according with SFA intake (G1 < 10% of TEE of TEE, G2 ≥ 10% of TEE of SFA).
Biochemical assessment
Blood samples were collected after an overnight fasting of 8 hours (ADA, 2008). Cholesterol, HDL and TG levels were analyzed by automated colorimetricenzyme method. LDL (LDL = cholesterol-HDL-TG/5) and very-low-density lipoprotein (VLDL) (VLDL = TG/5) was calculated.12 Reference values adopted to define the lipid profile of atherogenic risk were TG < 150 mg/dL, cholesterol < 200 mg/dL, HDL > 35 mg/dL and LDL < 100 mg/dL.13
The HbA1c determination was obtained by turbi-dimetry method certified by National Glycohemoglobin Standardization Program. HbA1c less than 7% are considered normal for diabetics. The serum glucose was analyzed by enzymatic colorimetric method. Values of fasting glucose recommended for diabetics from 90 to 110 mg/dL.1
Anthropometric and body composition assessment
The weight (kg) and height (m) were used to obtain the body mass index (BMI) (WHO, 1995)14 or BMI for age (WHO, 2006).15
Waist circumference (WC) was measured at the mean point between the lower rib and the iliac crest, at the moment of minimum respiration, using a SANNY flexible metal anthropometric tape measure with a 0.1-cm scale. WC was classified according to American Heart Association12 and International Diabetes Federation,16 adopting measurements for men and women over age 16 years > 94 cm and > 80 cm, respectively, with increased risk of metabolic complications.4
Body composition was assessed by bioelectrical impedance (Biodynamics model 450), which is based on the body resistance principle to passage of electric current in tissue hydrated, to obtain the values of total body water (TBW), lean body mass (LBM) and FM considering the two-compartment model.17
Dietary assessment
Was performed using the dietary records for 3 days (2 typical and 1 atypical day) to assess usual dietary intake. All records were analyzed using the nutritional software DietPró 5i. The composition of macronutrients and energy was evaluated. TEE was calculated for equations proposed by Food and Nutrition Organization.18
Statistical analysis
The data were expressed as mean values and standard deviation. Evaluated to normality of data distribution was made by Kolmogorov-Smirnov test. The t-test was used for non paired analysis between group. The Pearson correlation was used to describe the relationship between dietary, anthropometric and biochemical variables.
Analysis were performed in the SPSS 16.0 (Chicago, IL) statistical software considering a significance level at p < 0.05.
Results
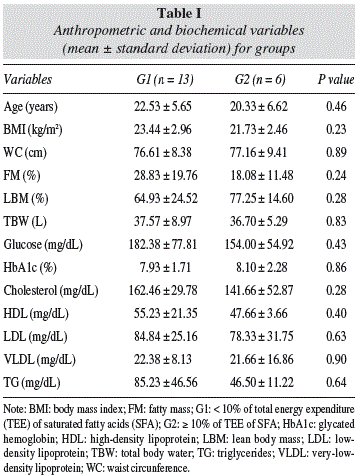
Table I shows the anthropometric and biochemical variables in G1 and G2, indicating normal BMI (18.5-24.9 kg/m2) in both groups. There was an increased in FM in G1 (28.83 ± 19.76%), but hadn´t difference between groups. G1 presented excess body fat.17 Lipemia did not differ between groups.
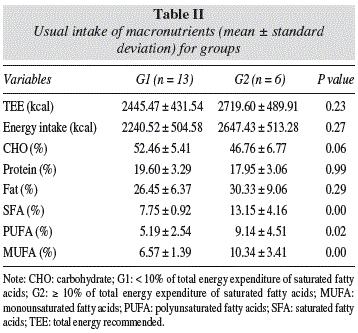
In both groups, the usual intakes are bellow of the TEE (WHO, 1995). Was a trend to lower CHO intake in G2 (46.76 ± 6.77%), compared G1 (52.46 ± 5.41%). However, the protein intake was similar between the groups (G1 = 17.96 ± 3.29% and G2 = 17.95 ± 3.06%), characterizing a normoprotean diet1. Fat intake in both groups was adequate (G1:26.45 ± 6.37%; G2 30.33 ± 9.06%), but G1 presented a SFA intake above recommended (≤ 10% to TEE)1 (table II).
MUFA intake by G1 (6.57 ± 1.39%) was low, but in G2 (10.34 ± 3.41%) was adequated1, and presented difference between groups (p = 0.01). G2 presented high PUFA intake, compared with G1, but both groups were eating according to recommendations1 (table II).
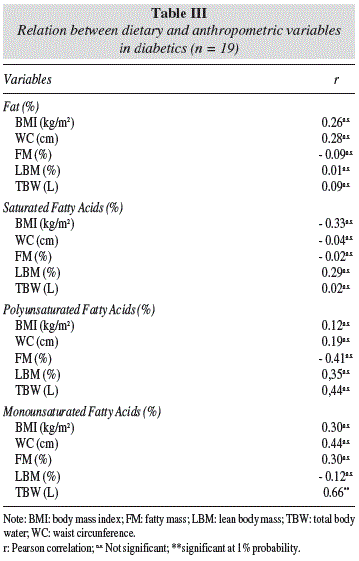
There were no significant relations between the total fat intake, SFA, PUFA and MUFA with anthropometric and biochemical variables except the MUFA intake were positively related to TBW (tables III and IV).
Discussion
Studies found that the type of fat diet is associated with obesity, independent of the amount of fat intake.19,20 Moussavi et al. (2008)20 showed that populations with lower prevalence of obesity, consumed a larger amount of MUFA, while PUFA and SFA were associated with a higher prevalence of obesity. Larson et al. (1996)21 observed in non-diabetic individuals, that SFA intake was positively related to FM, while PUFA intake were negatively associated with it.
Paniagua et al. (2007)22 and Puebla et al. (2003)23 observed positive effects of the MUFA intake in weight loss. The replacement of SFA by MUFA resulted in a significant weight and FM loss in men and women. The same studies have shown that in humans there is greater PUFA oxidation compared with SFA.
In the present study, the MUFA and PUFA intake was higher in G2 compared with G1, being that MUFA intake was inadequate in G1. G2 presented high SFA intake, but also adequated MUFA intake and this may have influenced the less FM. This relationship may be associated with the unsaturated fats intake have been around the recommendations proposed by the ADA. However, Doucet et al.,24 found that SFA and MUFA intake was associated with increased FM.
In our study was observed the positively association between MUFA and TBW, suggesting influence the type of fat dietary on body composition, whereas the TBW is inversely proportional to the FM.
HbA1c values in both the groups indicate risk for diabetic complications1 According to Delahanty et al. (2009),25 a high fat and SFA intake, and lower CHO intake was associated with a poor glycemic control in type 1 DM. In nondiabetic individuals the SFA intake was associated with increase HbA1C values.26 In the present study SFA intake did not influenced in HbA1C.
Dietary recommendations for patients with DM are similar to recommendations for non-diabetic subjects. However, in order to prevent CVD is necessary reduce SFA intake. The type of fat ingested is more important than the total amount in relation to risk of CVD.27,28 However, this relationship between SFA intake and increase LDL concentrations was not observed in our study. There were no difference in lipemia between groups.
It is suggested that patients with type 1 DM should be encouraged to adjust their diet in order to reduce the complications of the disease. Methods of assessing food intake must be constantly used to detect failure in diet and anthropometric and biochemical markers, that are important in monitoring the patient and to evaluate the response to nutritional therapy.
The type of fat ingested influence the body composition, but dos not affect lipemia and glycemia. The adequate MUFA intake may match the high SFA intake.
References
1. American Diabetes Association. Standards of medical care in diabetes: 2008. Diabetes Care 2008; 31 (1): S12-54. [ Links ]
2. World Health Organization. Diet, nutrition and the prevention of chronic diseases. Report WHO Consultation. Geneva: WHO; 2003. Technical Report Series, 916. [ Links ]
3. Stratton IM et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study (UKPDS 35). BMJ 2000; 321:405-12. [ Links ]
4. Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115: 114-126. [ Links ]
5. Nathan. Translating the A1C Assay Into Estimated Average Glucose Values. Diabetes Care 2008; 31: 1-6. [ Links ]
6. Ladeia AM, Stefanelli E, Frota CL, Moreira A, Hiltner A, Adan L. Association between elevated serum creative protein and triglyceride levels in Young subjects with type 1 diabetes. Diabetes Care 2006; 29 (2): 424-6. [ Links ]
7. Duarte ACG, Castellani FR. Semiologia nutricional. Rio de Janeiro: Axcel Books do Brasil; 2002. [ Links ]
8. Ahmed ML, Ong KKL, Watts AP, Morred DJ, Preece MA, DungerDB. Elevated leptin levels are associated with excess gains in fat mass in girls, but not boys, with type 1 diabetes: Longitudinal study during adolescence. J Clin Endocrinol Metab 2001; 86 (3): 1188-93. [ Links ]
9. Greefield JR, Samaras K, Chisholm DJ. Insulin resistence, intra-abdominal fat, cardiovascular risk factors, and androgens in health yung women with type 1 diabetes mellitus. J Clin Endocrinol Metab 2002; 87 (3): 1036-40. [ Links ]
10. Costa, PCA, Franco, LJ. Introdução da sacarose no piano alimentar de portadores de diabetes mellitus tipo 1 - sua influência no controle glicêmico. Arq Bras Endocrinol Metab 2005; 49 (3): 403-409. [ Links ]
11. Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual. Geneva: World Health Organization, 1991. [ Links ]
12. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem 1972; 18 (6): 499-72. [ Links ]
13. American Heart Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115: 114-126. [ Links ]
14. World Health Organization. Physical Status: the use and interpretation of anthropometry. WHO Technical Report Series no 854. Geneva, Switzerland: WHO, 1995. [ Links ]
15. World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length and body mass index-for-age. Methods and development. WHO (nonseral publication). Geneva, Switzerland: WHO, 2006. [ Links ]
16. International Diabetes Federation (IDF). The metabolic syndrome in children and adolescents: The IDF consensus. Diabetes Voice 2007; 52 (4): 29-32. [ Links ]
17. Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GL Assement of fat-free mass using bioelectrical impedance measurement of the human body. Am J Clin Nut 1985; 41 (4): 810-7. [ Links ]
18. Food and Nutrition Organization (FAO) Human energy requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Rome, 2001. [ Links ]
19. Ministerio de la Salud. Documento del consenso de Latinoamérica sobre la obesidad [homepage on internet], 1999, [access in 2006 Jun 23]. Available at: www.abeso.org.br/downloads.htm [ Links ]
20. Moussavi N, Gavino V, Receveur O. Is obesity related to the type of dietary fatty acids? An ecological study. Public Health Nutr 2008; 11: 1149-1155. [ Links ]
21. Larson DE, Ferraro RT, Robertson DS, Ravussin E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr 1995; 62 (4): 735-9. [ Links ]
22. Paniagua JA, Sacristana AG, Romero I, Vidal-Puig A, Latre JM, Sanchez E et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007; 30 (7): 1717-23. [ Links ]
23. Puebla RAF, Fuentes F, Martinez PP, Sanchez E, Paniagua JA, Miranda JL et al. A reduction in dietary saturated fat decreases body fat content in overweight, hypercholesterolemic males. Nutr Metab Cardiovasc Dis 2003; 13 (5): 273-7. [ Links ]
24. Doucet E, Alméras N, White MD, Després J-P, Bouchard C, Tremblay A. Dietary fat composition and human adiposity. Eur J Clin Nutr 1998; 52: 2-6. [ Links ]
25. Delahanty LM, Nathan DM, Lachin JL, Hu FB, Cleary PA, Ziegler GK et al. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr 2009; 89: 518-524. [ Links ]
26. Boeing H, Weisgerber UM, Jeckel A, Rose HJ, Kroke A. Association between glycated hemoglobin and diet and other lifestyle factors in a nondiabetic population: cross-sectional evaluation of data from the Potsdam cohort of the European Prospective Investigation into Cancer and Nutrition Study. Am J Clin Nutr 2000; 71 (5): 1115-1122. [ Links ]
27. Hu FB, Manson JE, Willett WC. Types of Dietary Fat and Risk of Coronary Heart Disease: A Critical Review. J Am Col Nutr 2001; 20(1): 5-19. [ Links ]
28. American Heart Association. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114: 82-96. [ Links ]
![]() Correspondence:
Correspondence:
Priscila Mansur Leal.
Instituto de Nutrição Josué de Castro.
Universidade Federal do Rio de Janeiro.
Brazil.
E-mail: priscila.unirio@gmail.com
Recibido: 11-II-2010.
1a Revisión: 8-X-2010.
Aceptado: 4-III-2011.