Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Nutrición Hospitalaria
versión On-line ISSN 1699-5198versión impresa ISSN 0212-1611
Nutr. Hosp. vol.33 no.2 Madrid mar./abr. 2016
https://dx.doi.org/10.20960/nh.92
TRABAJO ORIGINAL / Paciente crítico
Effect of glutamine on oxidative stress and inflammation in a rat model of fulminant hepatic failure
Efecto de la glutamina en el estrés oxidativo y la inflamación en un modelo de rata con insuficiencia hepática fulminante
Elizângela Gonçalves Schemitt1,4,5, Josieli Raskopf Colares2,4,5, Renata Minuzzo Hartmann1,4,5, María Isabel Morgan-Martins4,5, Cláudio Augusto Marroni6, M. Jesús Tuñón7 and Norma Possa Marroni1,2,3,4,5
1Graduate School of Medical Sciences. Federal University of Rio Grande do Sul. Porto Alegre, RS. Brazil.
2BioHealth Graduate Program. Lutheran University of Brazil. Canoas, Brazil.
3Graduate School of Physiology. Federal University of Rio Grande do Sul. Porto Alegre, RS. Brazil.
4Experimental Hepatology and Gastroenterology Laboratory, HCPA. Porto Alegre, Brazil.
5Oxidative Stress and Antioxidants Laboratory, ULBRA. Canoas, Brazil.
6Graduate School of Hepatology. Federal University of Health Sciences of Porto Alegre. Porto Alegre, Brazil.
7Institute of Biomedicine (IBIOMED) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). University of León. León, Spain
We would like to thank the Research Incentive Fund of the Clinical Hospital of Porto Alegre (FIPE/HCPA - project num-ber 12-0116) for the financial support, and the Coordination for the Improvement of Higher-Level Personnel (CAPES), the Research Support Foundation of Rio Grande do Sul (FAPERGS), the National Council of Research Development (CNPq), the Federal University of Rio Grande do Sul (UFRGS), the Experimental Hepatology and Gastroenterology Laboratory (HCPA/UFRGS) and the Lutheran University of Brazil (ULBRA).
ABSTRACT
Introduction: Fulminant hepatic failure (FHF) is a rare clinical syndrome, characterized by sudden and severe liver dysfunction. Thioacetamide (TAA) is a hepatotoxin whose administration can induce centrilobular necrosis in liver cells and increase the formation of reactive oxygen species and lipid peroxidation in rats. Glutamine is a precursor for glutathione synthesis.
Objective: The objective of the study to assess the antioxidant effects of glutamine in a rat model of TAA-induced FHF.
Methods: Male Wistar rats were divided into four groups according to treatment and time of assessment: control, glutamine (25 mg/kg), thioacetamide (400 mg/kg) and thioacetamide plus glutamine. Animals were assessed after 24, 36 and 48 hours. Blood samples were collected for the analysis of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), total bilirubin (TB) and creatinine (CRE) levels, and liver samples were used to evaluate lipid peroxidation, acid-reactive thiobarbituric substances (TBARS), antioxidant enzyme activity superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and glutathione S-transferase (GST). Nuclear factor κB (NF-κB), tumor necrosis factors (TNF-α) and nitric oxide synthase inducible (iNOS) levels were assessed by histology and immunohistochemistry.
Results: TAA caused alterations in biochemical and histological parameters, and increased markers of the inflammatory process. TBARS levels and the activity of SOD and GST were significantly lower in the glutamine group as compared to the TAA group. CAT activity was elevated in animals treated with glutamine as compared to the TAA groups. GPx activity was also lower in the glutamine-treated groups than in TAA-treated animals at 36 and 48 hours. Tissue damage and NF-κB, TNF-α and iNOS expression were significantly lower in animals treated with glutamine.
Conclusion: Glutamine has shown to have protective effects against liver damage in a rat model of TAA-induced FHF.
Key words: Hepatotoxicity. Lipid peroxidation. Antioxidants. Liver. Thioacetamide.
RESUMEN
Introducción: la insuficiencia hepática fulminante (IHF) es un síndrome clínico poco frecuente, que se caracteriza por una disfunción hepática severa y repentina. La tioacetamida (TAA) es una hepatotoxina cuya administración puede inducir necrosis centrolobulillar en las células hepáticas y aumentar la formación de especies reactivas de oxígeno y la peroxidación lipídica en ratas. La glutamina es un precursor para la síntesis de glutatión.
Objetivo: el objetivo del estudio es evaluar los efectos antioxidantes de la glutamina en un modelo de rata de IHF inducida por TAA.
Métodos: ratas macho Wistar se dividieron en cuatro grupos de acuerdo con el tratamiento y el tiempo de evaluación: control, glutamina (25 mg/kg), tioacetamida (400 mg/kg) y tioacetamida más glutamina. Los animales se evaluaron después de 24, 36 y 48 horas. Se recogieron muestras de sangre para el análisis de los niveles de aspartato aminotransferasa (AST), alanina aminotransferasa (ALT), fosfatasa alcalina (AP), bilirrubina total (TB) y creatinina (CRE), y muestras de hígado para evaluar la peroxidación lipídica, las sustancias reactivas al ácido tiobarbitúrico (TBARS), la actividad de las enzimas antioxidantes superóxido dismutasa (SOD), glutatión peroxidasa (GPx), catalasa (CAT) y glutatión S-transferasa (GST). Además se midieron mediante inmunohistoquímicael factor nuclear kappa N (NF-κB), el fator de necrosis tumoral (TNF-α) y la óxido nítrico sintasa inducible (iNOS).
Resultados: la TAA causó alteraciones en los parámetros bioquímicos e histológicos, y el aumento de los marcadores del proceso inflamatorio. Los niveles de TBARS y la actividad de SOD y GST fueron significativamente inferiores en los grupos de glutamina en comparación con TAA. La actividad de CAT se incrementó en los animales tratados con glutamina en comparación con la TAA. La actividad GPx también fue menor a las 36 y 48 h en los animales tratados com glutamina. El daño tisular y la expresión de NF-κB, TNF-α e iNOS fueron significativamente inferiores en los animales tratados con glutamina.
Conclusión: la glutamina ha demostrado tener efectos protectores contra el daño hepático en un modelo de IHF inducida por TAA en la rata.
Palabras clave: Hepatotoxicidad. Peroxidación lipídica. Antioxidantes. Hígado. Tioacetamida.
Introduction
Fulminant hepatic failure (FHF) is a rare clinical syndrome, characterized by severe and sudden liver dysfunction resulting in coagulopathy and encephalopathy in previously healthy individuals with no underlying liver disease. The condition can rapidly progress to coma and death by cerebral edema or multiple organ failure. FHF has a mortality rate of up to 85%, and, to date, can only be effectively treated by liver transplantation (1). FHF has been attributed to several etiologies, including hepatotoxic drugs, viral hepatitis and stroke (2). However, the mechanisms by which liver cells are destroyed and the processes responsible for liver regeneration are still unknown (3,4). Despite recent advances and technological improvements in intensive care medicine, the treatment of FHF remains among the most challenging issues in clinical practice (5).
Experimental models of toxin-induced hepatotoxicity have contributed to the elucidation of the physiopathology of several hepatic diseases, and to the identification and evaluation of many hepatoprotective agents. In rats, thioacetamide (TAA) has been found to induce FHF or cirrhosis, depending on the dosage and duration of exposure (6). TAA induces oxidative stress (OS) by increasing the formation of free radicals, which may cause extensive damage to proteins, lipids and deoxyribonucleic acid (DNA) molecules (7). The hepatotoxic effects of TAA are caused by S-dioxide (thioacetamide S-dioxide), an unstable reactive metabolite which induces necrosis and the formation of reactive oxygen species (ROS) through covalent bonding to macromolecules in the liver (1).
Hepatotoxicity triggers the activation of signaling molecules such as nitric oxide (NO) which have been implicated in the regulation of several physiological processes including vasodilation, inhibition of platelet aggregation, neurotransmission, neural plasticity and the modulation of inflammatory and immune responses (8). NO is synthesized from L-arginine by nitric oxide synthase (NOS), which exists in one of three isoforms, depending on the tissue analyzed: eNOS (endothelial NOS), nNOS (neuronal NOS) and iNOS (inducible NOS) (9). In most cells, iNOS expression is regulated by several cytokines, such as interleukins and tumor necrosis factors (TNF-α). TNF-α is a multifunctional cytokine which mediates acute phase responses in the liver, where it is regulated by nuclear factor kB (NF-κB), a transcription factor responsible for the induction of several genes involved in the expression of mediators and proinflammatory cytokines in response to several types of liver damage (8,10).
The human body employs several defenses against ROS, including antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione S-transferase (GST), and non-enzymatic antioxidants such as glutathione (GSH), alpha-tocopherol (vitamin C), and flavonoids. The function of these enzymes is to maintain low levels of ROS so as to avoid the production of excess free radicals (9). Antioxidants have been found to have an important role in protecting the liver from TAA-induced damage (7,11-13).
Glutamine is a free amino acid which accounts for approximately 60% of the amino acid content of the human body. Although this compound can be synthesized by the body under physiological conditions, a relative lack of glutamine may be observed under pathological conditions, leading to alterations in energy metabolism. Glutamine is also an important nutrient, and has been found to be involved in the reduction of liver ischemia, reperfusion injury, and alcohol-induced liver damage, in the protection from oxidative stress and in the reduction of inflammatory cytokine expression (14). The glutathione system plays a major role in reducing oxidative stress. Glutamine provides glutamate to this system in several tissues, such as liver and skeletal muscle, and has been shown to preserve total glutathione levels in hepatic and gut models (15). Glutamine may also have a protective effect against ischemia-reperfusion damage, alcohol-induced liver damage, ulcerative colitis and stomach damage resulting from portal hypertension (14,16-18).
Thioacetamide (TAA) is a hepatotoxin whose administration can induce centrilobular necrosis in liver cells (7,19), and lead to increased ROS formation and lipid peroxidation in rats. The investigation of these phenomena may make important contributions to the development of treatment strategies for these conditions. In the present study, a rat model of thioacetamide-induced FHF was used to investigate the antioxidant effects of glutamine and its associated mechanisms.
Material and method
Ethical considetrations
The present study was approved by the Health Research Ethics Committee of the Graduate School (GPPG) of the Clinical Hospital of Porto Alegre (project number 12-0116). All procedures were performed according to federal law 11.794 (20) and the European Council Directive regarding animal experimentation (21).
Animals
A total of 84 male adult Wistar rats (Rattus norvegicus albinus) with a mean weight of 250 g were kept in the Animal Experimentation Unit of the Clinical Hospital of Porto Alegre (UEA-HCPA) throughout the experiment, under 12 hour light/dark cycles at 20 oC to 25 oC and ad libitum access to food and water.
Induction of severe acute liver failure
Animals received two intraperitoneal injections of 400 mg/kg TAA (Sigma Chemical Co., St. Louis, MO, EUA), diluted in saline, at 8 hour intervals. This procedure was adapted from Shapiro et al. (6), De David et al. (7) and Rahman and Hodgson (22).
Glutamine administration
Powdered glutamine (Sigma Chemical Co., St. Louis MO, USA) was dissolved in saline and administered intraperitoneally at a dose of 25 mg/kg body weight, according to the protocol outlined below. The dose of glutamine was determined based on previous studies of its protective effects in experimental models of gastrointestinal damage (16,18).
Experimental Protocol
Animals were randomly assigned to one of three groups according to the time of assessment: 24, 36 or 48 hours (28 rats/group). Each group was then further divided into four subgroups (n = 7/group): CO (control group), G (glutamine control group), TAA (thioacetamide group) and TAA+G (glutamine-treated thioacetamide group). Animals in the CO group received 2 intraperitoneal injections of 0.9% NaCl solution at 8 hour intervals. Thioacetamide was administered intraperitoneally in two doses of 400 mg/kg given 8 hours apart. Glutamine was administered at a dose of 25 mg/kg ip half an hour after the second dose of TAA. Animals in the 36 hour-group received an additional dose of glutamine 24 hours after the beginning of the experiment, while those evaluated after 48 hours received a third dose of glutamine 36 hours after the beginning of the experiment, as shown in figure 1. At the end of each study period, rats were weighed and injected intraperitoneally with 95 mg/kg ketamine chlorohydrate and 8 mg/kg body weight xylazine chlorohydrate. Blood samples were collected from the retro-orbital plexus to assess hepatic integrity, and livers were removed for subsequent analysis. At the end of each study period, animals were killed by exsanguination under deep anesthesia.
Animal Survival
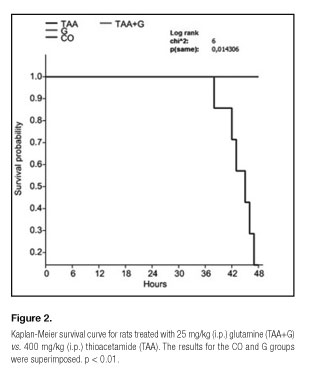
Animal survival was assessed 24, 36 and 48 hours after the administration of the first dose of TAA using a Kaplan-Meier curve and Log-rank testing for between-group differences.
Liver integrity analysis
Liver integrity was determined based on aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (AP) levels in the blood. Total bilirubin (TB) and creatinine (CRE) levels were also evaluated. Plasma AST (340 nm), ALT (340 nm), AP (405 nm), TB (546 nm) and CRE (510 nm) were determined by kinetic immunoassay using a Liquiform Labtest® commercial kit.
Histological and inmunohistochemical analysis
Histological analyses were performed on liver samples preserved in 10% formaldehyde solution for 24 h, which were then embedded in paraffin and cut in 3 μm slices using a rotating microtome. Histological examinations were performed using hematoxylin-eosin staining. Slides were analyzed under a binocular Labophot NIKON microscope, at 100 x magnification. Slides were assigned scores ranging from 0 to 3, where 0 = normal; 1 = mild damage with little inflammatory infiltrate; 2 = moderate damage and infiltrate, and 3 = intense damage and infiltrate with loss of hepatic architecture, as described by Shapiro et al. (6).
NF-κB, TNF-α and iNOS expression in liver tissue were determined by immunohistochemistry. Antigen epitopes were retrieved in citrate buffer at 100 oC. Endogenous peroxidase activity was inhibited by incubation in methanol. Slides were incubated overnight at 4 oC with monoclonal iNOS (Santa Cruz, 1:200), TNF-α (Santa Cruz, 1:200) or NF-κB antibody (Santa Cruz, 1:200), before being washed in buffer and incubated with a secondary antibody (rabbit anti-IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 minutes at room temperature. Images were acquired and processed using a microscope equipped with a digital camera and the Image-Pro Plus® software. NF-κB, TNF-α and iNOS expression were quantified by counting the number of positive pixels in the stained area using Adobe Photoshop CS3®. The level of expression was determined by multiplying the image density by the percentage of positively-stained (brown) nuclei.
Liver Homogenate Preparation
Livers were weighed and homogenized for 40 seconds in an Ultra-Turrax homogenizer (IKA-WERK) at 4 oC in the presence of 1.15% KCl (9 ml per g of tissue) and phenyl-methyl-sulfonyl fluoride (PMSF) at a concentration of 100 mM in isopropanol (10 µl per ml of KCl). The homogenate was then centrifuged for 10 minutes at 3,000 rpm in a refrigerated centrifuge (SORVALL Super T21; Condensed Operating Kendro Laboratory Products, USA). The precipitate was discarded and the supernatant was recovered and frozen at - 80 oC for subsequent biochemical analysis (23).
Protein
Protein concentrations were determined using the Bradford (24) method with bovine albumin as a standard (SIGMA), followed by spectrophotometry at 595 nm. Results were expressed in mg/ml, and used to determine TBARS (thiobarbituric acid-reactive substances) and antioxidant enzyme levels.
Lipid Peroxidation
The amount of aldehydes produced during lipid peroxidation was determined using TBARS as a marker. Samples were incubated at 100 oC for 30 minutes with 500 ml 0.37% thiobarbituric acid and 15% trichloroacetic acid, and centrifuged at 3,000 rpm for 10 minutes at 4 oC. Absorbance was determined by spectrophotometry at 535 nm (25).
Antioxidant Enzyme Analysis
Glutathione S-transferase (GST) activity was estimated based on the formation of glutathione conjugates of CDNB with absorption maximum at 340 nm. One unit of the enzyme catalyzes the formation of 1 mmol GSH per minute at 30 oC. Values were expressed as mmol/min/mg protein (26). Superoxide dismutase (SOD) activity was determined by the inhibition of the reaction of superoxide-dependent adrenaline auto-oxidation in a spectrophotometer at 480 nm. Results were expressed as USOD/min/mg protein (27). Catalase (CAT) activity was calculated based on the decomposition of hydrogen peroxide in a spectrophotometer at 240 nm. The results of this procedure were expressed as pmol/mg protein (28). Glutathione peroxidase (GPx) activity was quantified based on NADPH consumption and reduced oxidized glutathione levels, in a spectrophotometer at 340 nm, for 3 minutes. Measurements were expressed as nmol/mg protein (29).
Statical Analysis
Results were expressed as mean ± standard error. Statistical analysis was performed using Graphpad Instat v.3.0 or Prism v.6 for Windows. Survival curves were constructed using -Kaplan-Meier estimates, and analyzed using Log-rank tests. Correlations were evaluated by Pearson coefficients, and the remaining data were analyzed using analysis of variance (ANOVA) followed by Student's t tests and multiple comparison Student-Newman-Keuls tests, with significance set at 5% (p < 0.05).
Results
Survival Rates
All animals treated with glutamine after receiving thioacetamide survived until the end of the experiment (48 hours). However, those who received TAA only had a mortality rate of 85.7% (Fig. 2). In this group, no changes in mortality were observed after 24 or 36 hours.
Biochemical and liver integrity analyses
Exposure to TAA was associated with significant alterations in enzyme markers of liver damage (Table I). Animals injected with TAA showed increased TB, CRE, and liver damage marker levels at all assessment times. However, the peritoneal administration of 25 mg/kg glutamine led to significant reductions in AST, ALT, AP, TB and CRE levels.
Histology
Histological slides were stained with hematoxylin-eosin (HE) and analyzed at 100x magnification. Alterations in liver tissue were assigned a score between 0 and 3. Necrosis and inflammatory infiltrate scores were significantly higher in the TAA group as compared to controls, and lower in glutamine- than in TAA-treated animals after 24, 36 and 48 hours (Fig. 3A). In the absence of TAA, the liver parenchyma was preserved, hepatocyte strings were adequately arranged, nuclei were well-defined, and no inflammatory infiltrate was observed, as evidenced by the photomicrographs of the livers of control (Fig. 3B) and glutamine control animals (Fig. 3C). Figures 3C, 3D and 3F display photomicrographs of the liver of animals in the TAA group after 24, 36 and 48 hours, respectively. These images show extensive parenchymal damage, necrosis, and inflammatory infiltrate with polymorphonuclear cells. Figures 3G, 3H and 3I show the preservation of liver tissue and reduction in necrosis and inflammatory infiltrate after 24, 36 and 48 hours, respectively, in animals treated with glutamine.
NF-κB, TNF-α and iNOS expression
NF-κB (Fig. 4), TNF-α (Fig. 5) and iNOS (Fig. 6) levels did not differ between control and control + glutamine groups at any time of assessment. Animals exposed to TAA demonstrated lower staining for all proteins as compared to the control groups. Glutamine exposure led to significantly decreased protein expression in the TAA + G group.
Protein and lipid peroxidation measurements
Figure 7A shows a significant reduction in total protein levels in all animals exposed to TAA, and an increase in these values following the administration of glutamine. In contrast, the TBARS content of liver cells indicated increased lipid peroxidation in the TAA group as compared to the remaining animals, and decreased peroxidation in the TAA + G group as compared to the TAA group at all assessment times (Fig. 7B). The elevated TBARS levels in the TAA groups are suggestive of more severe liver damage. This finding was corroborated by the histological evidence of necrosis and inflammatory infiltrate shown in figure 8.
Antioxidant Enzymes
Figure 9 shows the results of antioxidant enzyme activity measurements. GST and SOD (Figs. 9 A and B) levels were significantly higher in the TAA group as compared to the remaining animals, and lower in TAA + G than TAA animals after 24, 36 and 48 hours. CAT (Fig. 9C) activity was significantly lower in the TAA group as compared to the remaining animals and higher in the TAA + G than in the TAA group at all three assessment times. GP x (Fig. 9D) activity was significantly lower in TAA animals as compared to the other groups after 24 hours, but was found to increase in TAA animals and decrease in the TAA+G group after 36 and 48 hours.
Discussion
Although several studies have attempted to develop experimental models of this condition, the mechanisms involved in the physiopathogenesis of FHF have not been well established (30). Thioacetamide is a highly toxic compound which can cause various degrees of liver damage in experimental animals, ranging from necrosis and hepatocyte disarray to cirrhosis (8,31). In the present study, glutamine was found to attenuate oxidative stress and inflammation in TAA-treated rats, similarly to previous findings of the effects of antioxidant molecules in animal models of FHF (32,33). TAA-administration led to severe liver damage and a high mortality rate after a 36- to 48-hour interval. In a study by Shapiro et al. (6) a mortality rate of 60% was observed after rats were administered 300 mg/kg TAA.
Histological analysis showed extensive necrosis and inflammation in the liver tissue of animals exposed to TAA, which points to the toxicity of the substance. Similar results have been obtained by other authors (6,7,31,34). Animals treated with glutamine exhibited a reduction in the parameters studied, demonstrating the protective effects of this substance. These findings are in agreement with those obtained by authors who assessed the protective effects of glutamine against gastrointestinal damage (16-18).
NF-κB is an important transcription factor required for the expression of several genes, especially those related to inflammation, interleukins and adhesion molecules (33). The TAA group showed increased expression of NF-κB, TNF-α and iNOS. Similar findings were reported by Shapiro et al. (6) and Son et al. (35). Glutamine decreased the expression of these proteins in liver tissue. An association between glutamine treatment and decreased expression of NF-B and TNF-B was also reported by Lin et al. (14).
TAA led to an increase in AST, ALT, AP, TB and CRE levels, suggesting the presence of liver damage. David et al. (7) also reported an increase in AST, ALT and AP levels in models of TAA-induced liver damage. Glutamine led to a reduction in the levels of these enzymes, pointing to its protective role against hepatocellular damage. Wu et al. (36) found that glutamine improved AST, ALT, AP, CRE and albumin levels in pigs exposed to the mycotoxin Deoxynivalenol.
TAA increased LPO by 69.7% after 24 hours, 162.07% after 36 hours and 124.24% after 48 hours. These results corroborate those obtained by De David et al. (7), who also performed experimental studies involving TAA-induced liver damage. Glutamine, on the other hand, decreased LPO by 36.36% after 24 hours, 68.97% after 36 hours and 30.30% after 48 hours. The decrease in TBARS following glutamine administration was also associated with a decrease in necrosis and inflammation scores at all assessment points (figure 8). These findings regarding the hepatoprotective role of glutamine were also reported by Marques et al. (18).
GST is a detoxifying enzyme which metabolizes xenobiotic substances, inactivating toxic metabolites by conjugation with reduced glutathione, thus protecting cells from exogenous and endogenous metabolites. In the present study, GST activity increased following the administration of TAA, but decreased following glutamine treatment. Similar findings were reported by Becker (37), who noted an increase in anion superoxide production following physical exercise in rats. In the present study, SOD activity increased by 325.38%, 111.86% and 360.96% after 24, 36, and 48 hours, respectively, in the TAA group, possibly in an attempt to compensate for the liver damage caused by the substance. Similar results were reported by Oliveira et al. (38), who studied the hepatotoxic effects of polychlorinated biphenyls (PCB) in rats. SOD levels were lowest in glutamine-treated animals at all assessment times (88%, 43% and 66%), corroborating the findings obtained by Ren et al. (39). Although CAT activity was consistently lower in TAA-treated animals, it increased following glutamine administration, as has been previously reported in the literature (31,38,40,41). GPx activity was lowest in the TAA group after the first 24 hours of the experiment, but exhibited an increase after 36 and 48 hours. In TAA + G animals, however, the measurements taken after 36 and 48 hours evidenced a decrease in GPx activity.
In animals injected with TAA, lipid peroxidation was caused by an increase in anion superoxide production, as evidenced by measures of SOD activity, which catalyzes the dismutation of superoxide into hydrogen peroxide and water. Although catalase degrades hydrogen peroxide, its activity was consistently low in TAA-treated animals, suggesting that, in this group, GPx may have been responsible for metabolizing ROS. Due to the toxic effects of TAA, GPx activity was still low at the 24-hour assessment, but increased at the 36 and 48-hour measurements, illustrating its hepatoprotective effects.
The present study confirmed the hepatotoxicity of TAA, as shown by its association with increased oxidative stress, elevated liver-associated enzymes, high levels of NF-κB and inflammatory cytokines, necrosis and inflammation. Glutamine appears to decrease oxidative stress and reduce the expression of inflammatory markers, making it a promising agent for reducing hepatocellular damage and treating liver toxicity.
References
1. Adukauskiene D, Dockiene I, Naginiene R, Kevelaitis E, Pundzius J, Kupcinskas L. Acute liver failure in Lithuania. Medicina (Kaunas) 2008;44(7):536-540. [ Links ]
2. San Miguel B, Crespo I, Álvarez M, Jorquera F, Tuñón MJ, González-Gallego J. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit hemorrhagic disease virus. J Pineal Res 2011;50:38-45. [ Links ]
3. Bantel H, Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol 2012;3:79. [ Links ]
4. Laliena A, San Miguel B, Crespo I, Álvarez M, González-Gallego J, Tuñón MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res 2012;53:270-278. [ Links ]
5. Demirel U, Yalniz M, Aygun C, Orhan C, Tuzcu M, Sahin K, et al. Allopurinol ameliorates thioacetamide-induced acute liver failure by regulating cellular redox-sensitive transcription factors in rats. Inflammation 2012;35(4):1549-1557. [ Links ]
6. Shapiro H, Ashkenazi M, Weizman N, Shahmurov M, Aeed H, Bruck R. Curcumin ameliorates acute thioacetamide-induced hepatotoxicity. J Gastroenterol Hepatol 2006;21(2):358-366. [ Links ]
7. David C, Rodrigues G, Bona S, Meurer L, Gonzalez-Gallego J, Tunon MJ, et al. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol 2011;39(6):949-957. [ Links ]
8. Kim YR, Lee NJ, Ban JO, Yoo HS, Lee YM, Yoon YP, et al. Curative effects of thiacremonone against acetaminophen-induced acute hepatic failure via inhibition of proinflammatory cytokines production and infiltration of cytotoxic immune cells and Kupffer cells. Evid Based Complement Alternat Med 2013;974794. [ Links ]
9. Hartmann RM, Morgan Martins MI, Tieppo J, Fillmann HS, Marroni NP. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig Dis Sci 2012;57(8):2038-2044. [ Links ]
10. Bautista M, Del Río MA, Benedi J, Sánchez-Reus MI, Morales-González JA, Téllez-López AM, et al. Effect of dichloromethylene diphosphonate on liver regeneration following thioacetamide-induced necrosis in rats. World J Hepatol 2013;5(7):379-386. [ Links ]
11. Li J, Li S, He B, Mi Y, Cao H, Zhang C, et al. Ameliorative effect of grape seed proanthocyanidin extract on thioacetamide-induced mouse hepatic fibrosis. Toxicol Lett 2012;213(3):353-360. [ Links ]
12. Wong WL, Abdulla MA, Chua KH, Kuppusamy UR, Tan YS, Sabaratnam V. Hepatoprotective effects of panus giganteus (Berk.) corner against thioacetamide- (TAA-) induced liver injury in rats. Evid Based Complement Alternat Med 2012;170303. [ Links ]
13. Singh S, Mondal P, Trigun SK. Acute liver failure in rats activates g-lutamine-glutamate cycle but declines antioxidant enzymes to induce oxidative stress in cerebral cortex and cerebellum. PLoS One 2014;9(4):e95855. [ Links ]
14. Lin Z, Cai F, Lin N, Ye J, Zheng Q, Ding G. Effects of glutamine on oxidative stress and nuclear factor-kappaB expression in the livers of rats with nonalcoholic fatty liver disease. Exp Ther Med 2014;7(2):365-370. [ Links ]
15. Prem JT, Eppinger M, Lemmon G, Miller S, Nolan D, Peoples J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg 1999;178(2):147-150. [ Links ]
16. Fillmann H, Kretzmann NA, San-Miguel B, Llesuy S, Marroni N, González-Gallego J, et al. Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology 2007;236(3):217-226. [ Links ]
17. Sozen S, Topuz O, Uzun AS, Cetinkunar S, Das K. Prevention of bacterial translocation using glutamine and melatonin in small bowel ischemia and reperfusion in rats. Ann Ital Chir 2012;83(2):143-148. [ Links ]
18. Marques C, Licks F, Zattoni I, Borges B, De Souza LE, Marroni CA, et al. Antioxidant properties of glutamine and its role in VEGF-Akt pathways in portal hypertension gastropathy. World J Gastroenterol 2013;19(28):4464-4474. [ Links ]
19. Díez-Fernández C, Sanz N, Cascales M. Changes in glucose-6-phosphate dehydrogenase and malic enzyme gene expression in acute hepatic injury induced by thioacetamide. Biochem Pharmacol 1996;51(9):1159-1163. [ Links ]
20. Brazil. Federal Law No. 11.794. Procedures for the scientific use of animals. Official Gazette. October 8, 2008. [ Links ]
21. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Communities 1986;(L358):1-28. [ Links ]
22. Rahman TM, Hodgson HJ. The effects of early and late administration of inhibitors of inducible nitric oxide synthase in a thioacetamide-induced model of acute hepatic failure in the rat. J Hepatol 2003;38(5):583-590. [ Links ]
23. Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4'-epiadriamycin in mice. Tumori 1985;71(3):241-249. [ Links ]
24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254. [ Links ]
25. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302-310. [ Links ]
26. Mannervik BG, Guthenberg C. Glutathione transferase. Methods in Enzymology 1981;77:731-735. [ Links ]
27. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-3175. [ Links ]
28. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 1973;134(3):707-716. [ Links ]
29. Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984;105:114-121. [ Links ]
30. Koblihova E, Mrazova I, Vernerova Z, Ryska M. Acute liver failure induced by thioacetamide: Selection of optimal dosage in Wistar and Lewis rats. Physiol Res 2014;4;63(4):491-503. [ Links ]
31. Salama SM, Abdulla MA, Alrashdi AS, Hadi AH. Mechanism of hepatoprotective effect of boesenbergia rotunda in thioacetamide-induced liver damage in rats. Evid Based Complement Alternat Med 2013;157456. [ Links ]
32. Crespo I, San Miguel B, Álvarez M, Culebras JM, González-Gallego J, Tuñón MJ. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res 2010;49:193-200. [ Links ]
33. San Miguel B, Álvarez M, Culebras JM, González-Gallego J, Tuñón MJ. N-acetyl-cysteine protects liver from apoptotic death in an animal model of fulminant hepatic failure. Apoptosis 2006;11:1945-1957. [ Links ]
34. Anbarasu C, Rajkapoor B, Bhat K, Giridharan J, Amuthan AA, Satish K. Protective effect of Pisonia aculeata on thioacetamide induced hepatotoxicity in rats. Asian Pac J Trop Biomed 2012;2(7):511-515. [ Links ]
35. Son G, Iimuro Y, Seki E, Hirano T, Kaneda Y, Fujimoto J. Selective inactivation of NF-kappaB in the liver using NF-kappaB decoy suppresses CCl4-induced liver injury and fibrosis. Am J Physiol Gastrointest Liver Physiol 2007;293(3):G631-639. [ Links ]
36. Wu L, Wang W, Yao K, Zhou T, Yin J, Li T, et al. Effects of dietary arginine and glutamine on alleviating the impairment induced by deoxynivalenol stress and immune relevant cytokines in growing pigs. PLoS One 2013;8(7): e69502. [ Links ]
37. Becker CU. Effect of aerobic exercise training on oxidative stress in skeletal muscle of rats with pulmonary hypertension. Graduate Program in Biological Sciences: Physiology. Porto Alegre: Federal University of the Rio Grande do Sul; 2013:78. [ Links ]
38. Oliveira CR, Ceolin J, Oliveira RR, Schemitt EG, Colares JR, Freitas LB, et al. Effects of quercetin on polychlorinated biphenyls-induced liver injury in rats. Nutr Hosp 2014;29(5):1141-1148. [ Links ]
39. Ren W, Yin J, Wu M, Liu G, Yang G, Xion Y, et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS One 2014;9(2):e88335. [ Links ]
40. Bona S, Filippin LI, Di Naso FC, De David C, Valiatti B, Isoppo Schaun M, et al. Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride-induced cirrhosis. Gastroenterol 2012;762920. [ Links ]
41. Marcolin E, Forgiarini LF, Rodrigues G, Tieppo J, Borghetti GS, Bassani VL, et al. Quercetin decreases liver damage in mice with non-alcoholic steatohepatitis. Basic Clin Pharmacol Toxicol 2013;112(6):385-391. [ Links ]
![]() Correspondence:
Correspondence:
Elizângela Gonçalves Schemitt.
Federal University of Rio Grande do Sul.
Porto Alegre, RS. Brazil
e-mail: elizschemitt@yahoo.com.br
Received: 16/11/2015
Accepted: 24/11/2015












 Curriculum ScienTI
Curriculum ScienTI