INTRODUCTION
Obesity has multiple causes, and it´s determined by the interaction between genetic factors and environmental 1. Especially, gene defects showing no or only minor effect when expressed alone might influence on the phenotype. A variant is the tryptophan-to-arginine (Trp64Arg) missense mutation in the beta 3 adrenoreceptor (Beta3-AR). Beta3-AR is the principle mediator of cathecolamine-stimulated thermogenesis and lipolysis, which mainly occur at subcutaneous and visceral sites 2. Trp64Arg variant in this receptor has been reported to be associated with increased body weight and insulin resistance 3.
The factors associated with cardiovascular disease (CVD) 4 include high blood pressure, high triglyceride levels, hyperglycaemia, low high-density lipoprotein (HDL), and obesity. Many of these risk factors are the ones that make up the so-called syndrome X or metabolic syndrome (MS) (5,6. One of the most accepted classifications for defining the metabolic syndrome is the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (ATP III) 7.
Moreover, adipose tissue is considered an active secretory organ, sending out and responding to signals that modulate appetite, insulin sensitivity, energy expenditure, and inflammation. Adipocytokines are proteins produced mainly by adipocytes 8. These molecules have been shown to be involved in the pathogenesis of the metabolic syndrome and cardiovascular disease.
The aim of our study was to investigate the relationship between metabolic syndrome and Trp64Arg polymorphism in the beta 3 adrenoreceptor gene in obese women.
SUBJECTS AND METHODS
SUBJECTS
A population of 531 (body mass index > 30) obese females patients was analyzed in cross-sectional survey. The recruitment of subjects was a non probabilistic method of sampling among patients send from Primary Care Physicians with obesity from Valladolid (norwest of Spain). Exclusion criteria included history of cardiovascular disease or stroke during the previous 36 months, malignant tumour or major surgery during the previous 6 months as well as the use of glucocorticoids, antineoplastic agents, and drinking and/or smoking habit. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the HURH ethics committee. Written informed consent was obtained from all patients and signed.
PROCEDURES
Weight, blood pressure, basal glucose, insulin, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides blood and citokines (leptin, adiponectin, resistin) levels were measured at basal time. Genotype of beta 3 adrenoreceptor gene polymorphism was studied. To estimate the prevalence of Metabolic Syndrome, the definitions of the ATPIII was considered 7. The cutoff points for the criteria used are three or more of the following; central obesity (waist circumference > 88 cm), hypertriglyceridemia (triglycerides > 150 mg/dl or specific treatment), hypertension (systolic BP > 130 mmHg or diastolic BP > 85 mmHg or specific treatment) or fasting plasma glucose > 110 mg/dl or drug treatment for elevated blood glucose
GENOTYPING OF BETA 3 ADRENORECEPTOR GENE POLYMORPHISM
Oligonucleotide primers and probes were designed with the Beacon Designer 4.0 (Premier Biosoft International(r), LA, CA). The polymerase chain reaction (PCR) was carried out with 250 ng of genomic DNA, 0.5 uL of each oligonucleotide primer (primer forward: 5'-CAA CCT GCT GGT CAT CGT-3'; primer reverse: 5'-AGG TCG GCT GCG GC-3'), and 0.25 uL of each probes (wild probe: 5'-Fam-CCA TCG CCT GGA CTC CG-BHQ-1-3') and (mutant probe: 5'-Hex-CAT CGC CCG GAC TCC G- BHQ-1-3') in a 25 uL final volume (Termociclador iCycler IQ (Bio-Rad(r)), Hercules, CA). DNA was denaturated at 95 °C for 3 min; this was followed by 50 cycles of denaturation at 95 °C for 15 s, and annealing at 59.3 °C for 45 s). The PCR were run in a 25 uL final volume containing 12.5 uL of IQTM Supermix (Bio-Rad(r), Hercules, CA) with hot start Taq DNA polymerase.
ASSAYS
Serum total cholesterol and triglyceride concentrations were determined by enzymatic colorimetric assay (Technicon Instruments, Ltd., New York, N.Y., USA), while HDL cholesterol was determined enzymatically in the supernatant after precipitation of other lipoproteins with dextran sulfate-magnesium. LDL cholesterol was calculated using Friedewald formula.
Plasma glucose levels were determined by using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, California). Insulin was measured by enzymatic colorimetry (Insulin, WAKO Pure-Chemical Industries, Osaka, Japan) and the homeostasis model assessment for insulin sensitivity (HOMA) was calculated using these values 9.
Adiponectin was measured by ELISA (R&D systems, Inc., Mineapolis, USA) with a sensitivity of 0.24 ng/ml and a normal range of 8.65-21.43 ng/ml, interassay coefficients of variation were less than 10%. Resistin was measured by ELISA (Biovendor Laboratory, Inc., Brno, Czech Republic) with a sensitivity of 0.20 ng/ml with a normal range of 4-12 ng/ml, interassay coefficients of variation were less than 10%. Leptin was measured by ELISA (Diagnostic Systems Laboratories, Inc., Texas, USA) with a sensitivity of 0.05 ng/ml and a normal range of 10-100 ng/ml, interassay coefficients of variation were less than 15%.
ANTHROPOMETRIC MEASUREMENTS AND DIETARY INTAKE
Body weight was measured to an accuracy of 0.1 kg and body mass index computed as body weight/(height2). Waist (narrowest diameter between xiphoid process and iliac crest) and hip (widest diameter over greater trochanters) circumferences to derive waist-to hip ratio (WHR) were measured, too. Tetrapolar body electrical bioimpedance was used to determine body composition (Biodynamics Model 310e, Seattle, WA, USA) 10. Blood pressure was measured twice after a 10 minutes rest with a random zero mercury sphygmomanometer, and averaged. Patients received prospective serial assessment of nutritional intake with 3 days written food records. All enrolled subjects received instruction to record their daily dietary intake for three days including a weekend day. Food scales and models to enhance portion size accuracy were used. National composition food tables were used as reference 11. Aerobic exercise was recorded in the same questionnaire.
STATISTICAL ANALYSIS
All the data were analysed using SPSS for Windows, verson 15.0 software package (SPSS Inc. Chicago, IL). Sample size was calculated to detect differences over 40% of prevalence of metabolic syndrome with 90% power and 5% significance. The results were expressed as average ± standard deviation. The statistical differences in genotype distribution and allele frequencies between groups and analysis of deviation from the Hardy Weinberg equilibrium were assesses usin chi-square or fisher exact test. Other variables were analyzed with ANOVA test (for normally-distribited variable) or Kruskal-Wallis test (for non-normally-distribited variable). The statistical analysis was performed for a dominant model. Logistic regression analyses were used to calculated odds ratio (OR) and 95% confidence interval (CI) to estimate the association of the rd161547 SNP with the risk of metabolic syndrome. A p-value under 0.05 was considered statistically significant.
RESULTS
Five hundred and thirty one patients gave informed consent and were enrolled in the study. The mean age was 45.9 ± 12.8 years and the mean BMI 36.5 ± 6.2.
Four hundred and sixty six patients (87.8%) had the genotype Trp64/Trp64 (wild group), whereas 65 (12.2%) had genotype Trp64/Arg64 (mutant group). Age was similar in both groups (wild type: 45.8 ± 12.3 years vs. mutant group: 46.1 ± 11.2 years:ns).
Prevalence of metabolic syndrome (MS) with ATP III definition was 47.1% (250 patients) and 52.9% patients without MS (n = 281 patients). Prevalence of beta 3 genotypes was similar in patients with metabolic syndrome (87.6% wild genotype and 12.4% mutant genotype) and without metabolic syndrome (87.9% wild genotype and 12.1% mutant genotype). Prevalence of each criteria of metabolic syndrome was calculated in wild and mutant type genotypes, without statistical differences. Elevated waist circumference was detected in 92.0% patients with wild type genotype and 94.8% patients with mutant type genotype. Elevated levels of triglycerides or specific treatment were detected in 20.4% patients with wild type genotype and 14.5% patients with mutant type genotype. Elevated levels of blood pressure or specific treatment were detected in 54.7% patients with wild type genotype and 54.7% patients with mutant type genotype. Elevated levels of glucose or specific treatment were detected in 33.6% patients with wild type genotype and 33.9% patients with mutant type genotype.
Table I shows the subjects´differences in anthropometric and cardiovascular variables with and without metabolic syndrome (MS). Females with MS had higher weight, BMI, waist circumference, waist to hip ratio, systolic and diastolic blood pressure, glucose, HOMA, insulin, total cholesterol, LDL cholesterol and triglycerides than patients without MS. Table II shows the subjects´levels of adipokines. Females with MS had lower adiponectin levels than patients without MS.
Table I Anthropometric and biochemical variables, metabolic syndrome vs. no metabolic syndrome
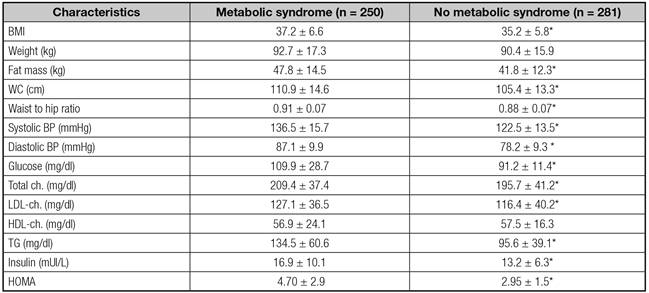
BMI: body mas index; Ch: cholesterol; TG: triglycerides; hOMA: homeostasis model assessment; WC: waist circumference. (*) p < 0.05, between groups.
Table II Circulating adypocitokines, metabolic syndrome vs. no metabolic syndrome

IL-6: interleukin 6. *p < 0.05, between groups.
Subject´s nutritional intake was similar in both groups (MS vs. no MS); calory (1757 ± 68 cal/day vs. 1689 ± 567 cal/day), carbohydrate (187.8 ± 68 g/day vs. 171.5 ± 73 g/day), fat (80.8 ± 35 g/day vs. 73.3 ± 29.3 g/day), protein (87.7 ± 24 g/day vs. 84.8 ± 25 g/day) and fiber intakes (14.9 ± 6.1 g/day vs. 14.1 ± 6.2 g/day). Hours of exercise per week were similar (1.54 ± 2.6 h/week vs. 1.37 ± 2.9 h/week), too. No differences in dietary intakes or physical activity were detected in both genotypes (wild vs. mutant type) in metabolic and no metabolic syndrome groups.
Table III shows the subjects´differences in anthropometric and cardiovascular variables secondary to genotype in metabolic and no metabolic syndrome. Patients with MS, in both genotypes, had higher weight, BMI, waist circumference, blood pressure, glucose, HOMA, insulin, and triglycerides than patients without MS. Significant differences in insulin and HOMA levels were detected between genotypes in the same group of metabolic syndrome. Insulin and HOMA levels were higher in patients with mutant genotype than wild type.
Table III Anthropometric and biochemical variables

MS: metabolic syndrome; BMI: body mas index; Ch: cholesterol; HOMA-IR: homeostasis model assessment; TG: triglycerides; WC: waist circumference; WT (wild type genotype Trp64Trp) MT (mutant type genotype Trp64Arg). +p < 0.05, statistical differences between MS and no MS groups in different allele groups (Trp64Trp vs. Trp64Arg). *Statistical differences between WT and MT in each allele group.
Table IV shows the subjects' levels of adipokines in both genotypes in metabolic and no metabolic syndrome. Patients with MS, in both genotypes had lower adiponectin levels than patients without MS. No differences in adipocytokines levels were detected between genotypes in the same group of metabolic syndrome.
Table IV Circulating adypocitokines

MS: metabolic syndrome; WC: waist circumference; WT (wild type genotype Trp64Trp) MT (mutant type genotype Trp64Arg)(*). +p < 0.05, statistical differences between MS and no MS groups in different allele groups (Trp64Trp vs. Trp64Arg). No statistical differences between WT and MT in each allele group.
DISCUSSION
The main finding of this study is the lack of association of the Trp64/Trp64 and Trp64/Arg64 genotypes with metabolic syndrome. Women of mutant genotype group of beta3 adrenoreceptor gene (Trp64/Arg64) have higher insulin and HOMA levels than wild type group, with and without metabolic syndrome.
Our present finding that the frequency of the Arg64 allele was 12.2% in obese female's patients agrees with previous reports 12,13. Secondly, a meta-analysis assessing quantitative phenotypes in relation to Trp64Arg polymorphism with BMI across diverse populations did not find evidence of this association 14, as our study shows.
Thirdly with respect to insulin resistance, omental adipocyte beta 3 adrenoreceptor sensitivity was related to waist hip ratio and insulin resistance in subjects with Arg allele of this polymorphism 15. Perhaps, defects in beta 3 adrenoreceptor signal transduction, binding, or regulatory mechanism may result in a disminished lipolytic response in visceral adipose tissue, aggravating insulin resistance. Our data showed metabolic differences between wild and mutant type patients, with increased levels of insulin and HOMA in females with and without metabolic syndrome. The mechanism through which the Arg64 variant alters insulin sensitivity could be explained by adipocytokine actions, too. Resistin and adiponectin appear to be important in regulating insulin sensitivity 16). Leptin is another adipocytokine that has been implicated in glucose homeostasis. It is thought to have some role in regulating insulin sensitivity 17, too. The mechanism through which adipocytokines alters sensitivity cannot be explained from our data, because we did not observe differences among adipocytokines in wild and mutant type groups. The decreased levels of adiponectin in our patients with metabolic syndrome are expected. Adiponectin decreases lipid synthesis and glucose production in the liver and causes decreases in glucose and free fatty acid concentrations in the blood. In offspring of diabetes mellitus type 2 patients 18.
Other previous study has demonstrated that obese postmenopausal women who are heterocygous for this beta 3 adrenoreceptor variant had greater insulin resistance than women homozygous for the normal gene 19. Kurabayashi et al. 20 showed that females heterozygous for this variant, but not men, have clinical manifestations of obesity and insulin resistance syndrome. Our results have been confirmed by Gjesing et al. 21, too. In this study, the Trp64 polymorphism was not associated with obesity, but, the Arg allele was associated with increased insulin resistance and insulin levels. Therefore, the lack of association with BMI and waist circumference as a measure of obesity may reflect that these measures do no adequately represent the biological body fat related variable influenced by the Trp64Arg variant.
Perhaps, these unclear results in the literature 22-24 may partially explain by differences in ethnic background, baseline BMI, gender distribution, previous weight loss, experimental design (early stage or late stage type 2 diabetes mellitus), accuracy of measures of insulin, and basal adypocitokines levels of participants. Therefore, interaction between gene and ambient could explain these differences with bias in previous studies.
Nevertheless, the cross sectional design of our study showed the lack of association of this polymorphism with metabolic syndrome. Therefore, interaction between gene and environmental factors, such as dietary treatments 25,26, could influence in development metabolic syndrome in the following years of these patients
In conclusion, in mutant group of beta3 adrenoreceptor gene (Trp64/Arg64) patients have higher insulin and HOMA levels than wild type group, without relation with metabolic syndrome. Further studies are needed to explore this unclear topic area 27.














