INTRODUCTION
There have been several recent studies focusing on caloric intake in parenteral nutrition (PN) while protein content relevance has been underestimated. Protein deficiency has also an impact on patient outcomes 1) (2.
There is a clear need to identify safe amounts of nutrients for each acute disease stage in order to avoid under or overfeeding 3 . In critically ill patients, undernourishment has been correlated with a higher number of infections, respiratory and immunology function impairment, as well as an increase in hospital length of stay and mortality 4. The results of a European study in 2012 including 102 patients showed a higher mortality rate on those patients who received a lower protein load, less than 1.2-1.5 g/kg/day 5. Other recent trials have shown that most critically ill patients do not reach nutritional requirements, being a low protein intake the most remarkable one 5) (6) (7) (8) (9, although there are some exceptions 10.
Based on our experience, we wanted to design a study to quantify the nitrogenous content in parenteral nutrition (PN) during 2010-2013 periods, comparing patients in the general ward and in the Intensive Care Unit. At the same time, we recorded the number of standardized/tailored solutions as well as insulin and glutamine addition.
MATERIAL AND METHODS
A retrospective descriptive study was performed. The pharmacist recorded daily, clinical, anthropometric and analytical information using the Nutridata(r) program. PN composition was also recorded. This program offers the possibility to explore data through SPSS(r). Some content parameters, considered as targets, were obtained to estimate differences among different groups of patients.
Some of the analyzed variables were current weight, present weight, ideal weight (IW) and the % of weight loss/time. Body mass index (BMI) was calculated after exporting weight and height information to Excel, as well as the adjusted weight (AW = IW ± 25% of the excess or defect in weight). Current weight data are the result of the analysis done through the Process Infornut(r) 11, when a nutritional risk alarm arises in the filter FILNUT 12) (13, during the hospital stay. If there was no opportunity to record the patient's weight, it was obtained through digital clinical records (Diraya specialized care - DAE - oncology pharmacy - Farmis Oncofarm(r), reports from preanesthesia, hemodynamics, etc.). In any case, it is mandatory to fulfill patient's weight and height into the justification sheets for PN 14. Any missing information would be obtained through the visit done by a member of the nutritional team. When a new weight is recorded into Nutridata(r) from a patient on long-term PN, the mean weight is used as the calculator for global weight/case. When it is not feasible to get patient's height, it can be estimated from the ulna bone length. Variables such as g N/kg or the non-protein kcal/g N are calculated by the program on a daily basis. The percentage and number of PN diets and their distribution as per the different target variables were calculated using the Nutridata(r) database per case and day. The information regarding g N/kg is referred to the current weight in the four-year period. Enteral nutrition intake was not considered.
Three groups of patients were established: general ward, ICU, and ICU requiring renal replacement therapy (RRT). Patients on RRT are those who received PN at any time during the RRT therapy, even if it was for a short period of time.
In 2011, the Nutritional Support Team proposed a new PN protocol. It included 27 different diets ranging from standard, fat-free, cholestasis, renal o liver failure and sepsis mixtures with different nutrient content. As a novelty, it also included diets with 18 and 20 g N with a low non-protein kcal/g N ratio (highly stressed patients) (Smofkaviben(r) and Olimel(r) triple chamber bags); glutamine dipeptide (Dipeptiven(r)) was added in certain cases, mainly in critically ill patients. A personalized diet was defined as the one with different macronutrients and/or volume composition when compared with those in the protocol, but not different in terms of electrolytes, insulin addition or glutamine composition.
The one-way analysis of variance (ANOVA) test was used after checking data for normality and homoscedasticity.
RESULTS
Table I shows results in the three groups. The most severe and the longer the stay, the longer the length of PN use, and the higher the nitrogen load. The number of tailored formulations was higher in ICU patients requiring RRT. Table II shows yearly evolution in the study period. The number of PN bags remains similar, with a trend to higher nitrogen content and lower nitrogen/non-protein calorie ratio.
Table I Comparison of nitrogen content in patients according to their location
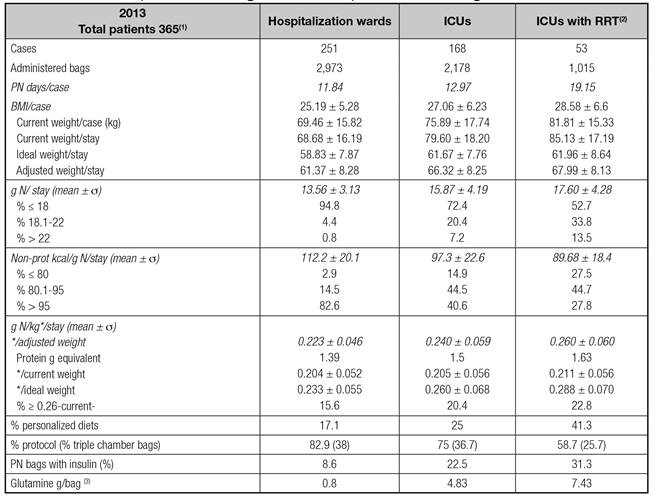
1. Fifty-four patients stayed both in ICUs and wards; 731 bags were excluded (five homecare patients). 2. Critically ill patients requiring RRT at any time. It includes all bags with or without RRT. 3Equivalent to 6.1; 36.6 and 56.3 ml of Dipeptiven(r)/bag.
Table II Nitrogen content evolution between 2010 a 2013

1. Hospitalization wards, ICUs and homecare patients included. 2. Personalized: those different from the protocol in terms of macronutrients and/or volume, not those with added electrolytes and/or insulin or glutamine dipeptide.
Figure 1 shows the evolution of the nitrogen intake as per weight, in the four-year period and other indicators fixed as targets. Figure 2 shows average values/stay (day) and 95% CI for nitrogen (g), non-protein kcal/g N, adjusted weight (AW) and g N/Kg AW in the three groups of patients. Patients in the ICU requiring RRT received a significantly higher nitrogen load as well as a lower N/non-protein kcal ratio.
DISCUSSION
Standard PN solutions are widely used in most hospitals. There is also the possibility of designing standardized formulations based on standard patient profiles, including different stress situations, sepsis, cholestasis, and renal or hepatic impairment. Individualized formulations could be of interest in those patients with a complex clinical situation (up to 25-40% of the cases) 15. The ready-to-use bags (triple chamber bags) have dramatically reduced the need for manual compounding, by significantly reducing manipulation and liquid transfer 16. Martínez Romero et al. 17 demonstrated that 75% of their metabolically stable patients had their needs addressed by only three standardized formulations they had designed according to case-mix. Schoenenberger et al. showed that a standardized protocol which included 20 diets helped to prepare 6,300 formulations; up to 30% of the cases were prepared from different macronutrients, although only 8% of the formulations were really individualized 18.
As our own program allowed collecting data on prescription, elaboration and dispensation, we were able to assess the protein load in our PN solutions in all three scenarios: general ward, ICU patients, and those in the ICU requiring RRT. There was an increase in the g N/stay year after year (p < 0.01) except from 2012 and 2013 (p = 0.181). The range was established from 4.1 to 32.6. PN (%) diets with ≥ 18 g N have increased every year, ranging from 12.8% (2010) to 19.6% (2013). Data from 2014 show the same trend (21.3% ≥ 18 g N). There were no yearly significant differences for mean weight or BMI per case. Nevertheless, the mean weight/stay on PN in 2013 was significantly higher (p < 0.001) than in the three previous years.
In Weijs paper, including 886 patients, an optimal caloric and protein intake (between 1.2 and 1.5 g/kg/day) was associated with a decrease in mortality, while only reaching the caloric targets was not sufficient to establish that association 19. In agreement with this, in a pilot study including 2,772 ICU patients a higher caloric and protein intake was associated with better clinical outcomes, especially if BMI was < 25 o ≥ 35 on admission 20. Other studies confirm these findings 21) (22, suggesting that classically recommended protein intake (ESPEN) may not be sufficient.
Regarding the use of glutamine, most published studies in critically ill patients have shown beneficial effects 23, leading to diverse scientific societies to recommend glutamine use 24) (25. Heyland described that very high doses administered through enteral and parenteral route (> 0.5 g/kg/day) and during the acute phase in patients with multiple organ dysfunction and shock increased mortality 26.
According to recent recommendations, we have increased the g N/stay in our PN from 14 to 15.05, with a decrease in the non-protein caloric content, from 112 to 102. This nitrogen increase has happened despite the increase on PN average duration (two days) and partly due to the increase in the number of complementary PN, but also as a result of an improvement on the way PN is gradually initiated in patients at risk of refeeding syndrome. There was a yearly significant increase in g N/kg/stay from 0.20 to 0.22 until 2012 (p < 0.001). In 2013 there was a significant decrease when compared with 2012. The value reached was similar to 2011 value (0.21) and higher than 2010 value. This could be explained by the higher current weight/stay in 2013. Its equivalent in g prot/kg/d varied between 1.26 and 1.38, in accordance with ESPEN guidelines for PN 27.
The reduction in non-protein kcal/g of N turns out to be significant (p < 0.001 year by year, excepting 2011-2012, p < 0.05). An important reduction appeared in this ratio, lowered from 111.6 (2010) to 101.8 (2013). The need for personalized diets was more necessary (26.5% in 2013) when the nitrogenous content increased.
We also found a significant difference among groups when comparing the variable g N/stay (13.5 vs 15.9 vs 17.6, p < 0.0001). The same significance was reached for the difference in non-protein kcal/g N/stay (112 vs 97 vs 90). The increase in g N/kg/stay in ICU did not reach statistical significance when compared with hospitalization wards; however, those on RRT did (p < 0.01). These differences were statistically significant among the three groups when the variable g N/kg considered the IW or AW/stay (p < 0.0001). Patients on the general wards received 0.22 by average, while the ICU ones received 0.24. Those on RRT received 0.26 g N/kg AW, raising up to 0.29 g N when referred to the IW.
Critically ill patients, and those requiring RRT, had higher weight than those in hospital wards, received PN for a longer period, and also received higher nitrogenous content, in absolute terms and with regards to weight kg, and less non-protein calories per gram. Finally, glutamine dipeptide, as a PN component, was mainly used in these patients.
If a clinical pharmacist is fully integrated in the interdisciplinary nutrition support team, as it was in our case, in close collaboration with the Intensive Care Unit as a reference consultant, it is more feasible to adjust nutrient supplies to patient needs and, therefore, to favor quality of care in patients requiring PN.
















