INTRODUCTION
The industrialization of food processing in the twentieth century paralleled with loss of quality have given rise to increased palatability, digestibility, overconsumption and the current obesity epidemic 1. Containment of the obesity epidemic is compounded by the addictive properties of sugar which involve the dopamine receptors 2. Adolescents are the highest consumers of sugar sweetened drinks 3, and factors linked to glucose metabolism are involved in the etiology of several cancers. High glycemic index (GI) or high glycemic load (GL) diets, which chronically raise postprandial blood glucose, may increase cancer risk 4. In spite of this, cancer is actually the principal cause of death in children under 15 years old 5. Metabolic syndrome (MS), an important problem of the childhood in Mexico, is another kind of pathology that befalls on young patients with cancer 6. Today in Mexico, oncological diseases are the second leading cause of death in the general population. Official statistics reported 80% of prevalence diagnoses patients on treatment survive to malignant tumors 7. Chemotherapy is a useful tool to attack cancer and mellow down the sufferings which it entails, and cytarabine is the most common chemotherapeutic agent used in children 8, although its use is accompanied by elevation of monoamine concentrations in the brain regions 9. Guidelines on sugar consumption by the World Health Organization (WHO) recommend non-sugar sweeteners such as stevia and splenda as healthy sugar replacement alternatives 10. Recently, the cultivation of Stevia rebaudiana Bertoni has gained interest for its potential use as a non-caloric sweetener with antioxidant properties 11.
Some studies suggest that chemotherapeutic agents such as cytarabine induce the interaction of proteins and activating factor (GcMAF) that inhibit cancer cell proliferation and metastatic potential, and may lead to nitric oxide (NO) release 12.
Since free radicals are known to damage cell components 13, mainly plasma membrane lipids 14, nitric oxide is a neuromodulator; however, an extra amount may lead to cell damage by oxidative stress or by forming nitroso-glutathione (NOGSH) within the cell 15. The central nervous system (CNS) intervenes in the control of food consumption and free radicals (FR) and actively participates in the metabolic functions of the same 16. It has been demonstrated that many biological processes are influenced by mechanical changes in membrane lipid components 17, and regulates energy and glucose homeostasis by acting on hypothalamic neurocircuits and higher brain circuits such as the dopaminergic system 18. Plasma membrane phospholipids in brain are in close contact with structural proteins that are embedded in the lipid bilayer 19, and through which the ionic interchange is maintained by the action of Na+, K+ ATPase that stimulates Na+ and K+ flows 20. The inhibition of the Na+, K+ ATPase activity induces excitatory amino acids release within the central nervous system 21.
Based on the above backgrounds, the purpose of the present study is to compare the protective effect of stevia and splenda on the levels of dopamine and 5-HIAA monoamines, and selected oxidative stress markers in brain regions of young rats treated with cytarabine.
METHODS
Forty-eight young male Wistar rats each with a weight of 80 g (four weeks old), distributed in six groups of eight animals each, were treated as follows: group 1, control (NaCl 0.9% vehicle); group 2, cytarabine (0.6 g/kg); group 3, stevia (0.6 g/kg); group 4, cytarabine + stevia; group 5, splenda; and group 6, cytarabine + splenda. Cytarabine was given intravenously (IV) while stevia and splenda were administered orally for five days, using orogastric tube. At the end of treatment, the animals were sacrificed and glucose levels in blood were measured. The animals were sacrificed by decapitation at the end of the treatment, and their brains were immediately dissected in cortex, striatum, and medulla/oblongata and immersed in a solution of NaCl at 0.9% and maintained at 4 °C. In the last day of treatment, blood samples were obtained and used to measure the levels of glucose. Each brain region was homogenized in 3 ml of tris-HCl 0.05 M pH 7.2 and used to determine lipid peroxidation (TBARS), Na+, K+ ATPase activity, levels of glutathione (GSH), serotonin metabolite (5-HIAA) and dopamine, using previously validated methods. The samples were kept at -20 °C until their assessment and others were stained with eosin-nigrosin to evaluate the histological abnormalities. Rats were procured from the Bioterium of the Instituto Nacional de Pediatría of Mexico City, and housed eight per cage in clean plastic cages and allowed to acclimatize in the room environment for one day. Animals were maintained in a mass air displacement room with a 12-h light/12-h dark cycle at 22 ± 2 °C with a relative humidity of 50 ± 10%. Balanced food (Rodent diet 5001) and drinking water were given to the animals ad libitum before and during study. The study protocol was previously approved by the Committee of Laboratory Animals Care of the National Institute of Pediatrics. Besides, all experimental procedures were performed following the national and
international guidelines for animal care.
TECHNIQUE TO MEASURE BLOOD GLUCOSE
The measurement of blood glucose was carried out at the end of the treatment. Two blood samples (20 µl each) were drawn from the tail-end without anticoagulant and placed on Accu-Chek(r) (Roche Mannheim, Germany) equipment reactive paper. The blood glucose concentrations were measured and reported in mg/dl.
MEASUREMENT OF DOPAMINE
The levels of dopamine were measured in the supernatant of tissue homogenized in HClO4 after centrifugation at 9,000 rpm for ten minutes in a microcentrifuge (HettichZentrifugen, model Mikro 12-42, Germany), with a version of the technique reported by Calderón et al. 22. An aliquot of the HClO4 supernatant, and 1.9 ml of buffer (0.003 M octyl-sulphate, 0.035 M KH2PO4, 0.03 M citric acid, 0.001 M ascorbic acid), were placed in a test tube. The mixture was incubated for five minutes at room temperature in total darkness, and subsequently the samples were read in a spectrofluorometer (PerkinElmer(r) LS 55, England) with 282 nm excitation and 315 nm emission lengths. The FL WinLab version 4.00.02 software was used. Values were inferred in a previous standardized curve and reported as nMoles/g of wet tissue.
MEASUREMENT OF 5-HYDROXYINDOL ACETIC ACID (5-HIAA)
5-HIAA levels were measured in the supernatant of tissue homogenized in HClO4 after centrifugation at 9,000 rpm for ten minutes in a microcentrifuge (HettichZentrifugen, model Mikro 12-42, Germany), with a modified version of the technique reported by Beck et al. 23. An aliquot of the HClO4 supernatant, and 1.9 ml of acetate buffer 0.01M pH 5.5 were placed in a test tube. The mixture was incubated for five minutes at room temperature in total darkness, and subsequently, the samples were read in a spectrofluorometer (PerkinElmer(r) LS 55, England) with 296 nm excitation and 333 nm emission lengths. The FL WinLab version 4.00.02 software was used. Values were inferred in a previously standardized curve and reported as nM/g of wet tissue.
MEASUREMENT OF GLUTATHIONE (GSH)
GSH levels were measured from the supernatant of the homogenised tissue, which was obtained after centrifuging at 9,000 rpm during five minutes (Mikro 12-42 centrifuge, Germany) based on a modified method of Hissin and Hilf 24; 1.8 ml phosphate buffer pH 8.0 with EDTA 0.2%, 20 μl taken from the supernatant and 100 ml of ortho-phthaldehyde at 1 mg/ml in methanol were mixed in a test tube and incubated for 15 min at room temperature in absolute darkness. Following the incubation, the mixture was spectrophotometrically read in PerkinElmer(r) LS 55, with excitation and emission wavelengths of 350 and 420, respectively. FL WinLab version 4.00.02 software was used. Values were inferred from a previous standardized curve and expressed as nM/g.
MEASUREMENT OF TOTAL ATPase
The activity of ATPase was assayed according to the method proposed by Calderón et al 25; 1 mg (10%) w/v of homogenised brain tissue in tris-HCl 0.05 M pH 7.4 was incubated for 15 minutes in a solution containing 3 mM MgCl2, 7 mM KCl, and 100 mM NaCl. To this, 0.5, 1, 2, 3, and 4 mMtris-ATP were added and incubated for another 30 min at 37 °C in a shaking water bath (Dubnoff Labconco); 100 µl 10% trichloroacetic acid w/v was used to stop the reaction, and samples were centrifuged at 100 g for five minutes at 4 °C. Inorganic phosphate (Pi) was measured in duplicates using one supernatant aliquot as reported by Fiske and Subarrow 26. The absorbance of a supernatant sample was read at 660 nm in a Helios-, UNICAM spectrophotometer and expressed as mM Pi/g wet tissue per minute.
MEASUREMENT OF LIPID PEROXIDATION (TBARS)
Determination of TBARS was carried out using the modified method of Gutteridge and Halliwell 15, as described below: from the homogenized brain in tris-HCl 0.05 M pH 7.4, 1 ml was taken, and 2 ml of thiobarbaturic acid (TBA) which contains 1.25 g of TBA, 40 g of trichloroacetic acid, and 6.25 ml of concentrated chlorhydric acid (diluted in 250 ml of deionized H2O) were added. Samples were heated to boiling point for 30 minutes (Thermomix(r) 1420), after which they were immersed in ice bath for five minutes and, finally, centrifuged at 700 g for 15 minutes (Sorvall(r) RC-5B Dupont). The absorbance of the supernatants tissues was read in triplicate at 532 nm in a spectrophotometer (Helios-, UNICAM). The concentration of reactive substances to thiobarbaturic acid (TBA-RS) was expressed as µM of malondialdehyde/g of wet tissue.
HISTOLOGICAL ANALYSIS IN BRAIN REGIONS
Histological examination of the tissue was conducted after immediately following brain extraction. The tissues were gently rinsed with a physiological saline solution (0.9% NaCl) to remove blood and adhering debris. Brains were taken and fixed in a 10% neutral-buffered formalin solution for 24 h. The fixed specimens were then trimmed, washed and dehydrated in ascending grades of alcohol. These specimens were cleared in xylene, embedded in paraffin, sectioned at 4-6 mm thickness and stained with hematoxylin and eosin (H&E) and then examined microscopically 27.
STATISTICAL ANALYSIS
Analysis of variances (ANOVA) and Kruskal-Wallis test were used with their corresponding contrasts and previous variance homogeneity comparison. Values of p < 0.05 were considered as statistically significant 28. The JMP version 8.0.0 for academic was used.
RESULTS
The levels of blood glucose discretely increased in each of the treatments administered in the study groups without statistically significant difference (Fig. 1).

Figure 1 Levels of glucose in blood of young rats treated with cytarabine and sweeteners. Mean values ± SD.
Dopamine concentration in cortex decreased significantly in the group treated with stevia + cytarabine when compared with those that received stevia alone (Fig. 2). Compared with the rest of the groups, this bioamine did not register significant differences. In striatum, an important reduction in the concentrations of dopamine was observed in all the groups when compared with the control group. The comparison among the groups treated with splenda or splenda + cytarabine did not show any differences; however, in the groups treated with stevia or stevia + cytarabine, a significant reduction in the later could be appreciated. In cerebellum/medulla oblongata, a high significant reduction in dopamine concentration was found in the groups that received cytarabine, splenda, splenda + cytarabine and stevia + cytarabine with respect to the control. With the exception of the group treated with stevia, the same behavior was seen in those which were administered cytarabine. When the stevia group was compared with the stevia + cytarabine group, there was a significant decrease of this bioamine in the later.
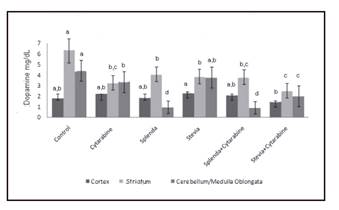
Figure 2 Levels of dopamine in brain regions of young rats treated with cytarabine and sweeteners. Levels not connected by same letter are significantly different. Cortex: Kruskal-Wallis 2 = 11.78, p = 0.037; striatum: Kruskal-Wallis 2 = 52.04, p < 0.0001; cerebellum/medulla oblongata: Kruskal-Wallis 2 = 110.47, p < 0.0001.
In cortex, differences in the concentration of 5-HAA among the study groups were not observed (Fig. 3). In striatum, all the groups showed a significant reduction in the concentration of the bioamine when compared with the control. In cerebellum/medulla oblongata, a significant decrease was seen in the groups treated with splenda, splenda + cytarabine and stevia + cytarabine with respect to the control, while the same behavior was appreciated in the group that received splenda or splenda + cytarabine when compared with the group that was administered cytarabine alone.

Figure 3 Levels of 5-HAA in brain regions of young rats treated with cytarabine and sweeteners. Levels not connected by same letter are significantly different. Cortex: Anova F = 1.23, p = 029; striatum: Kruskal-Wallis 2 = 30.99, p < 0.0001; cerebellum/medulla oblongata: Kruskal-Wallis 2 = 60.98, p < 0.0001.
The activity of GSH witnessed a significant decrease in the groups treated with splenda and splenda + cytarabine in the cortex, while in striatum the GSH activity reduced significantly in the group treated with splenda + cytarabine when compared with those that exclusively received splenda (Fig. 4). When comparing the activity of this bioamine among the group that received splenda + cytarabine with the control or cytarabine or splenda alone, a significant decrease was seen, while the comparison stevia vs stevia + cytarabine did not show any differences in cerebellum/medulla oblongata.
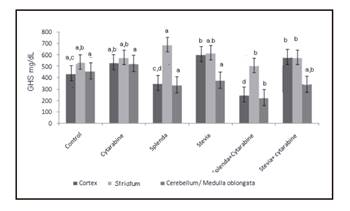
Figure 4 Levels of GSH in brain regions of young rats treated with cytarabine and sweeteners. Levels not connected by same letter are significantly different. Cortex: Anova F = 21.50, p = < 0.0001; striatum: Anova = 2.29, p < 0.048; cerebellum/medulla oblongata: Kruskal-Wallis 2 = 29.30, p < 0.0001.
Lipid peroxidation in cortex reduced significantly in all the groups with respect to the control, and this was more evident in the group that received stevia (Fig. 5). In striatum, the same pattern was observed except in the group that received splenda + cytarabine. In cerebellum/medulla oblongata, a decrease in lipid peroxidation was seen in the groups with cytarabine, stevia and stevia + cytarabine in comparison with the control. The comparison of splenda vs splenda + cytarabine and stevia vs stevia + cytarabine did not show any differences in the levels of the bioamine.
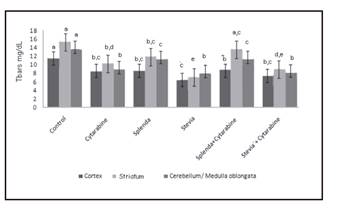
Figure 5 Levels of Tbars (lipid peroxidation) in brain regions of young rats treated with cytarabine and sweeteners. Levels not connected by same letter are significantly different. Cortex: Anova F = 21.50, p = < 0.0001; striatum: Anova = 2.29, p < 0.048; cerebellum/medulla oblongata: Kruskal-Wallis 2 = 29.30, p < 0.0001.
ATPase activity in cortex and in all the groups witnessed a light increase with respect to the control. This increase was statistically significant in the groups treated with splenda, splenda + cytarabine and stevia + cytarabine (Fig. 6). Comparison among the groups treated with the antineoplasic or the sweeteners did not show significant differences. The bioamine showed significant increased activity in the group that received cytarabine + splenda when compared with animals treated with cytarabine only.

Figure 6 Levels of ATPase activity in brain regions of young rats treated with cytarabine and sweeteners. Levels not connected by same letter are significantly different. Cortex: Anova F = 9.62.
In striatum, there was no difference in the activity of the enzyme. However, in cerebellum/medulla oblongata, a significant reduction in the ATPase activity was observed in the group treated with stevia when compared with the control. When comparing the activity of the enzyme in the group that received only cytarabine with those treated with cytarabine + sweetener, a statistically significant difference was observed only in those that received stevia and stevia + sweetener.
HISTOLOGICAL ANALYSIS
The control animals' brains presented a normal cytoarchitecture, the well-defined form of the cellular and nuclear membrane being evident, with thin chromatin and homogenous neuropile in the three analyzed structures, cortex, striatum and cerebellum/medulla oblongata (Fig. 7A-C).
The treatment with cytarabine caused evident damage in the same structures analyzed. In the cortex (Fig. 7D), a reduction in cell size, loss of shape and evident pyknosis were observed in a significantly high number of cells (arrow head). In the striatum (Fig. 7E) the damage was slightly lower and cells were preserved, although the damage is evident, presenting cellular retraction and vacuolization in addition to pyknosis and loss of definite form (arrow head). In the cerebellum/medulla oblongata of rats treated with cytarabine (Fig. 7F) the same type of cell damage was found (arrow head).

Figure 7 Micrographs of cortex (A), striatum (B) and cerebellum/medulla oblongata (C) of control rats, where normal cells are seen, with well-defined, rounded cysts, nucleus with evident thin chromatin. In cortex (D), striatum (E) and cerebellum/medulla oblongata (F) of animals treated with cytarabine, morphological alterations such as pyknotic cells characterized by gradual acidophilia, evidenced by darkening of the nucleus and cytoplasm, contraction of the pericarp and loss of the boundaries of cytoplasmic membranes, spindle cells and angled edges (arrow heads), can be observed.
In the panels (Fig. 8G-I) corresponding to cortex, striatum and cerebellum/medulla oblongata of rats treated with the splenda sweetener, there is slight cellular damage and the cytoarchitecture is very similar to that of the controls. In the cerebellum/medulla oblongata (Fig. 8I) we find more damaged cells than in cortex and striatum, without becoming significant (arrow head).
In the cortex, striatum and cerebellum/medulla oblongata sections of animals co-administered with splenda and cytarabine (Fig. 8J-L), damage is apparently similar to that of animals treated only with cytarabine (Fig. 7D-F), so we think that the sweetener splenda had no effect on those animals.

Figure 8 Micrograph of cortex (G), striatum (H) and cerebellum/medulla oblongata (I) of animals treated with splenda sweetener. Cells with normal morphology were found in cortex and striatum (G and H), as well as in the cerebellum/medulla oblongata a slight damage was found with cells in the degeneration process (I, indicated by the arrow). In J, K and L cells, corresponding to cortex, striatum and brain stem of animals co-administered with splenda and cytarabine respectively, damaged cells are seen, although slightly smaller than in the cells of animals treated with cytarabine alone.
The analyzed structures of animals treated with the stevia sweetener (Fig. 9M-O for cortex, striatum and cerebellum/medulla oblongata respectively) do not present evident damage. In cortex, striatum and cerebellum/medulla oblongata of animals co-administered with stevia plus cytarabine (Fig. 9P-R) there are pyknotic cells with angled borders, cellular retraction and neuropile a little evident. However, minor damage is observed in cortex when it is compared with the cortex of animals treated only with cytarabine (Figs. 9P vs 7D), which suggests a slight protective effect of stevia against damage induced by cytarabine in the cortex. Striatum and cerebellum/medulla oblongata of animals with co-administration of stevia with cytarabine (Figs. 9Q and R) have a similar damage produced only with cytarabine.

Figure 9 Micrograph of cortex (M), striatum (N) and cerebellum/medulla oblongata (O) of animals administered with stevia sweetener. Normal cytoarchitecture with homogeneous distribution and well defined forms in cortex and striatum are observed. In figure O, which corresponds to cerebellum/medulla oblongata, some cells in the process of degeneration are appreciated, but nucleus and nucleolus are still visible. Cortex (P) striatum (Q) and cerebellum/medulla oblongata (R) of rats with co-administration of stevia and cytarabine also showed cells damaged in the process of degeneration, but in a smaller number than those treated only with cytarabine.
DISCUSSION
Consumption of sugar-sweetened beverages may be one of the dietary causes of metabolic disorders 29. In the present study, the sweeteners slightly increased the glucose levels in blood of animals that received cytarabine, suggesting hypoinsulinemia effects.
Precursors of neuroactive substances can be obtained from dietary sources, which can affect the resulting production of such substances in the brain 30. The brain levels of dopamine in males can be controlled by an intake of tyrosine in food, and chronic food restriction induces dopamine conservation 31. However, in the present study dopamine increased in cortex and decreased in striatum of animals that received stevia alone or combined with cytarabine, suggesting changes in the hypothalamus for the control of energy homeostasis and within the brain regions related with rewards 32. These results were contrary to those of Abhilash et al. 33, who found that other artificial sweeteners as aspartame decreased dopamine in corpus striatum and cerebral cortex, and serotonin in corpus striatum.
With respect to 5-HIAA levels, precursor of serotonin, it decreased in striatum and cerebellum/medulla oblongata of animals that received sweeteners and cytarabine alone or combined. These findings coincide with those of El-Merahbi et al. 34, who pinpointed that peripheral serotonin has an impact on the regulation of the function of the organs involved in glucose and lipid homeostasis. Indeed, peripheral 5HT plays an important role in the regulation of glucose homeostasis through the differential expression and activation of 5-HT membrane receptors on the surface of hepatocytes, adipocytes and pancreatic -cells 35.
GSH concentration increased in striatum of animals that received sweeteners (splenda and stevia) and decreased with the cytarabine administration. These results suggest neuroprotection and antioxidant effects, and coincide with previous reports of stevia, a diterpenic carboxylic alcohol with three glucose molecules, mainly used as a substitute for non-alcoholic sweetener 10.
Lipoperoxidation levels decreased in cortex, striatum and cerebellum/medulla oblongata regions in groups that received sweeteners and cytarabine. This effect of neuroprotection could be due to polar compounds obtained during the extraction of compounds like chlorophylls, carotenoids, phenolic and flavonoids involved in stevia and splenda. These substances are not physiologically inert compounds and their consumption may have potential biological mechanisms which may impact on energy balance and metabolic function 36.
Ca2+, Mg2+ ATPase activity increased in cortex and striatum regions, and decreased in cerebellum/medulla oblongata of animals that received sweeteners and cytarabine. These results suggest that glucose concentration, which mimics fasting, decreased intracellular NADPH and increased Na(+) concentration in single arcuate nucleus neurons, of the body's energy state, which subsequently exhibited Ca(2+) responses to lower glucose 37.
CONCLUSIONS
These results show sweeteners as stevia or splenda may lead to the onset of unfavorable changes in biogenic amines, dopamine and 5-HIAA, independent of significant effects of cytarabine administration. Antioxidant effects may be involved. Besides, histological changes revealed marked lesions of neuronal cells in experimental animals treated with cytarabine compared to those in the normal controls. Further studies are needed to confirm the opposing effects of high dietary glucose levels on risks of cancer.














