INTRODUCTION
Life expectancy has increased steadily in developed countries during the last century and the health status of people aged ≥ 65 years is therefore of growing importance. This age group is known to present increased risk of several diseases that are associated with greater use of health resources. Currently, older age is not a contraindication for surgical procedures 1,2 or for admission to intensive care units (ICU) 3, but older patients do experience more surgical complications and have increased risk of adverse outcomes 1,3. Older age is also not a contraindication for parenteral nutrition (PN) 4. To our knowledge, no specific studies have been published on the prevalence of PN support in older patients in comparison to younger patients in general hospitals. In a retrospective-cohort study on critically-ill adult patients in 125 USA hospitals, PN usage declined slightly and similarly, around 6%, over time in all age groups, including elderly patients, during an eight-year period 5. A cross-sectional study on PN use over three months in 15 acute care hospitals in the north of England found that patients receiving PN had a median age > 65 years 6. Thus, older patients seem to be exposed to PN in at least the same proportions as younger patients. However, few studies have assessed the effect of PN in the elderly compared to young patients 7,8. These retrospective studies did not find differences in nutritional and inflammatory markers, biochemical outcomes and associated morbidity of PN when compared to younger controls. However, older patients may present metabolic alterations during PN as diminished plasma lipoprotein-lipase activity 9, limited capacity to mobilize fat stores 10, and lower glucose oxidation 11 or have comorbidities that could increase PN complications 4.
The main purpose of this study was to assess metabolic complications of PN, comparing the incidence of adverse events (hyperglycemia, hypertriglyceridemia, and liver function test [LFT] alterations) in non-critically ill adult inpatients receiving PN between those patients ≤ 64 years and those ≥ 65. A secondary purpose was to compare the incidence, as a first appearance, of any of these three adverse events, as a variable of overall metabolic complications between the two groups.
MATERIAL AND METHODS
A retrospective analysis of a prospectively collected data set was performed on a cohort of medical and surgical non-critically ill adult inpatients receiving PN in 15 hospitals in Catalonia (Spain) from January 1st 2014 to December 31st 2014. The study was reviewed and approved by the ethics committees of Hospital Universitari Sagrat Cor, Parc de Salut Mar, and Hospital Clínic de Barcelona, Spain. The rest of the hospitals adhered to these approvals.
PATIENTS
Patients were classified into two groups depending on age: group YOUNG included adult patients aged between 35 and 64 years (middle age and older adulthood), and group OLD, senior patients aged between 65 and 95 years (younger and older old age). They were followed-up until PN was discontinued for any reason or oral or enteral nutrition started, or they were admitted to ICU or to Post-Anesthesia Care Unit (PACU) for three days or longer, or were transferred to another hospital.
INCLUSION AND EXCLUSION CRITERIA
Patients who entered the study met the following inclusion criteria: non-critically ill, recent weight available, and PN duration longer than six days. In addition, PN, including peripheral PN containing lipid emulsions, had to be the unique source of nutritional support covering at least 80% of the equation-calculated resting energy expenditure (cREE) 12.
The following acute conditions at the beginning of PN were exclusion criteria: sepsis; hypovolemic or cardiogenic shock; renal failure with a calculated glomerular filtration rate (cGFR) < 30 ml/min/1.73 m2 13; triglyceridemia > 250 mg/dl; any LFT (total bilirubin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma glutamyl transferase [GGT], or alkaline phosphatase [ALP]) value > 1.5-fold the upper limit of normal range (ULN); a glycemia test > 180 mg/dl 24 hours before the beginning of PN; biliary or hypertriglyceridemia-induced pancreatitis; and admission due to hyperosmolar hyperglycemia, diabetic ketoacidosis, or hypoglycemia under 70 mg/dl. Chronic conditions considered as exclusion criteria were: chronic liver disease, hepatobiliary disease, diabetes mellitus type 1, insulin-treated diabetes mellitus type 2, and body mass index (BMI) > 35 kg/m2. Patients were also excluded if they underwent biliopancreatic or liver surgical procedures, or they were admitted for longer than three days to ICU or PACU within the first 72 hours of PN course.
VARIABLES
Main outcomes were the comparison of the incidence of hyperglycemia, hypertriglyceridemia, and LFT alterations between the two groups defined above. The secondary variable was defined as the combined variable of the presence of any of the above-mentioned complications (hyperglycemia, hypertriglyceridemia, or LFT alterations).
Hyperglycemia was defined as capillary or plasma glycemia ≥ 180 mg/dl. If it was registered by capillary test, it had to be measured at least twice daily. Hypertriglyceridemia was defined as plasma triglycerides > 400 mg/dl. LFT alteration was defined as any liver enzyme increase > 2-fold the ULN, conjugated bilirubin > 2 mg/dl, or total bilirubin > 2.5 mg/dl. Blood clinical chemistry parameters were obtained at least once weekly.
DATA COLLECTED
At the beginning of the study, data collected were demographics (sex, age, and BMI), main diagnosis, ward area (medical, surgical or trauma), date of surgical procedure if relevant, Charlson comorbidity index 14, indication for and date of PN initiation, and cREE calculated by the Mifflin-St Jeor equation 12. Additional data were registered when any alteration in main variables was detected and at the end of PN: amounts of macronutrients (amino acids, glucose, and lipids) delivered in PN, and the ratio of kilocalories provided by PN and cREE; biochemical parameters (glycemia, triglyceridemia, albumin, creatinine, total bilirubin, conjugated bilirubin, AST, ALT, GGT, ALP); ICU or PACU admission for three days or longer; the reason for PN discontinuation when it occurred, and PN duration. As overall health outcomes, hospital length of stay (LOS) and mortality during hospital admission were recorded.
As the co-administration of some medications might alter the variables studied, the number of medications affecting glycemia, triglyceridemia, or LFTs was collected when they were administered for at least three days during PN. A list of medications to be monitored relating to hyperglycemia (nine medications) 15, hypertriglyceridemia (12 medications) 16, and LFT alterations (53 medications) was obtained from several sources 17,18,19.
PARENTERAL NUTRITION
PN was prepared following usual hospital practices as an "all-in-one" admixtures containing amino acids, glucose and lipid emulsions. They were administered in a 24-hour perfusion through central catheters or peripheral lines.
STATISTICAL ANALYSIS
Descriptive statistics were reported by age category. Continuous variables were reported as mean ± standard deviation (SD), and categorical variables were reported as frequency and percent. To assess for equivalency between groups, continuous variables (age, Charlson comorbidity index, BMI, kilojoules from PN) were compared using Student's t-tests. The categorical variables (sex and admission diagnosis category) were compared using the Chi-squared test or Fisher's exact test.
Unadjusted incidence rates of complication outcomes (hyperglycemia, hypertriglyceridemia, and LFT alterations) were reported by age group. Using an adjusted Cox proportional hazard model, age categories were compared with regard to each complication outcome assessed (hyperglycemia, hypertriglyceridemia, and LFT alteration). Potential factors associated with the occurrence of each complication outcome studied were evaluated by the use of univariate analysis. For each complication assessed, variables with a two-tailed p-value below 0.10 in the univariate analysis or those that were deemed clinically relevant were selected for inclusion in a multivariate Cox proportional hazard model to identify variables associated with occurrence of the complication evaluated. The final model was determined using backward elimination variable selection (Wald statistic). The initial model contained all the independent variables selected.
In the same way, the secondary outcome was assessed by adding all potential factors found in a multivariate Cox proportional hazard model to identify variables associated with the occurrence of any of the complications studied.
Additional analyses were performed to compare the rate of patients free of any adverse event over time (Kaplan-Meier plot) between groups.
In all analyses, p values were two-tailed, and a p < 0.05 was considered as statistically significant. SPSS Version 12 statistical software for Microsoft Windows (SPSS Inc., Chicago, IL, USA) was used for all analyses.
RESULTS
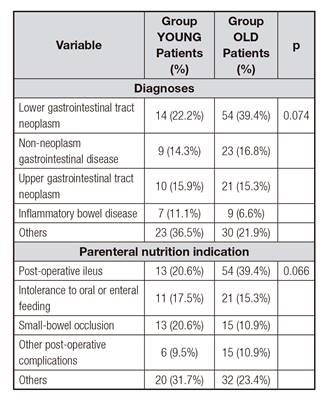
This study recruited 200 patients. Group YOUNG included 63 (31.5%) patients and group OLD, 137 patients (68.5%). Both groups differed in several baseline parameters (Table I and Table II). Group OLD presented lower cREE, cGFR, triglycerides, cholesterol, and albumin, but higher Charlson comorbidity index. This group received smaller amounts of macronutrients, but the same ratio of kilocalories provided/cREE during a similar period.
Table II Baseline patient parameters and PN characteristics

ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; cGFR: calculated glomerular filtration rate; GGT: gamma glutamyl transferase; cREE: resting energy expenditure.
Hyperglycemia was detected in 37 (18.5%) patients, eight (12.7%) in group YOUNG, and 29 (21.2%) in group OLD (p = 0.174). Surgical patients showed greater rates of hyperglycemia and LFT alterations than non-surgical patients. Hypertriglyceridemia appeared in only one (0.7%) patient in group OLD. LFT alterations were detected in 141 (70.5%) patients, 44 (69.8%) in group YOUNG and 97 (70.8%) in group OLD (p = 1.000). Patients with LFT alterations showed differences in baseline cGFR, albumin, and ALP, and amounts of glucose and amino acids administered compared with those without alterations. Complication outcome incidence rates and differences found are listed in Table III.
Table III Complication outcome incidence rates

*For the analysis, the presence of more than one medication affecting liver function tests or glycemia, depending on the statistical test, was considered. Variables presented as value (%) or value (±) depending on the variable shown. ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; cGFR: calculated glomerular filtration rate; GGT: gamma glutamyl transferase.
Table IV Hazard ratios (HRs) for occurrence of liver function test alteration, hyperglycemia and any complication

Presented as hazard ratio (95% confidence interval). *Medications related to hyperglycemia. †Medications related to liver alterations. ‡Medications related to any of the events assessed (liver alteration or hyperglycemia).
Table IV shows hazard ratios for hyperglycemia and LFT alterations, both adjusted for age group, surgical procedure, and medication use. Multivariate Cox models were additionally adjusted for differences found: for the hyperglycemia model, and for diabetes mellitus type 2 and amount of glucose administered. For the liver-function-test-alteration model, adjustments were for baseline FA, albumin, cGFR and amount of macronutrients administered. The final model for hyperglycemic events included diabetes mellitus type 2, surgical procedures, and use of medications that increase glycemia. For LFT alterations, the final model included surgical procedures, amounts of lipid and amino acid administered, use of more than one medication related to liver alteration, and age group.
The Cox proportional hazards regression for predictor variables on secondary outcome (the incidence of any of the three adverse events) found three variables as significant predictors: surgical procedure, diabetes mellitus type 2, and use of medications causing hyperglycemia, hypertriglyceridemia, or LFT alterations (Table IV). Amino acid amounts were also included in the final model. Since only one patient showed hypertriglyceridemia, no statistical analysis was performed for this complication.
Adverse events were detected at day 6.01 ± 3.99 without differences between groups (p = 0.952). Hyperglycemia appeared at day 4.68 ± 4.45; the only hypertriglyceridemia was detected at day 7 and LFT alterations appeared at day 6.79 ± 4.12.
Amongst those patients who suffered adverse events, patients of group OLD had the same number of days of PN (12.91 ± 7.48 vs 13.85 ± 6.66; p = 0.462), LOS (27.01 ± 16.41 vs 28.11 ± 13.82; p = 0.691) and mortality (10/104 vs 2/4; p = 0.343) as those of group YOUNG.
There was no difference in the rates of patients free of any adverse event over time between the two groups (p = 0.808) as shown by the Kaplan-Meier curve in Figure 1.
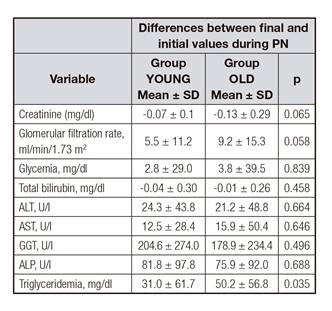
There were no differences between groups in the rates of incidence of variables from the beginning to the end of PN except for triglyceridemia, which increased more in group OLD (Table V).
DISCUSSION
In our study, older patients did not present a greater incidence of metabolic complications during PN than younger patients. In addition, older age was not related to the appearance of adverse events. In our patients, the main predictors for the adverse events studied were surgical procedures, diabetes mellitus type 2, and the concomitant use of medications related to metabolic disorders.
Our findings were in accordance with previous studies reporting no differences between older and younger non-critically ill patients receiving PN 7,8. Nonetheless, differences were found on baseline characteristics as well as amount of nutrients administered between the two groups assessed. However, differences found have been assessed with regression models in order to manage them.
In an early study, aging was reported to increase glycemia during PN in a heterogeneous cohort including critically-ill patients 20 but rates of hyperglycemia were not assessed. Diminished insulin response in the injured elderly might have contributed to this finding 20. In a study on a cyclic 6-hour PN regimen with high energy low protein content, glucose oxidation was about 30% lower and glycemia was about 20% higher with comparable insulinemia in elderly patients when compared with middle-aged patients 11.
In a more recent study to determine the prevalence of diabetes, prediabetes, stress hyperglycemia, and factors affecting hyperglycemia (> 180 mg/dl) in non-critically ill patients receiving PN, age ≥ 65 years showed a significant relationship to the development of hyperglycemia compared with younger age groups 21. However, age was not found to be a factor for hyperglycemia in the present study maybe because we excluded diabetes mellitus type 1 and insulin-treated diabetes mellitus type 2 patients. This discrepancy could be also related to the glucose doses received. Older and younger patients received similar glucose loads in these former studies. In our study, older patients received about 0.3 g of glucose/kg/day and about 2.18 kcal/kg/day less than younger patients. This is consistent with the fact that older patients have less cREE than younger patients 22,23, but our older patients received the same ratio of kcal provided/cREE as younger ones (Table II).
Studies on fat oxidation rates have shown inconclusive results in older adults compared to younger ones 23.
Specifically, older patients presented roughly 30% higher lipid oxidation, 35% higher FFA blood levels, and a lower respiratory quotient, but comparable triglyceridemia levels 11. In other study, older subjects presented higher FFA blood levels, about 65% lower lipoprotein lipase activity, and about 15% lower fat oxidation rates than younger patients, but triglyceridemia remained comparable in both groups. The authors concluded that elderly subjects can metabolize intravenous fat emulsions similarly to young individuals 9. In our study, at the end of PN, older patients showed higher triglyceridemia than younger patients while receiving a fat load of around 0.8 g/kg/day (Table V), but only one hypertriglyceridemic episode was detected. Our findings seem to be in agreement with the aforementioned studies, showing that older patients can maintain triglyceridemia levels similarly to younger patients, despite differences in fat oxidation. In contrast, in other study hypertriglyceridemia was detected in 36% of older patients (≥ 80 years) versus 8% in younger patients (< 80 years), but the authors did not show nutritional loads and mean triglyceridemia. Additionally, they defined hypertriglyceridemia as > 168 mg/dl whilst we set the cut-off point at 400 mg/dl in accordance with current European guidelines 24 and PN hypertriglyceridemia literature 25. The influence of age has not been studied extensively in PN in association with liver alterations in adults. Reilly et al. found no differences in maximum values of ALT, ALP, and bilirubin in patients < 80 years when compared to older patients. However, the group of older patients represented only 13% of the entire cohort 8. In a study using a cumulative logit model and including 162 patients receiving home PN for > 6 months, age was not a relevant factor in the development of liver disease 26. In another study on home PN, age was not a factor for chronic biochemical cholestasis in a cohort of 113 patients 27. Additionally, age was not a factor for liver dysfunction associated with specialized nutrition support in a prospective cohort of 725 critically ill patients 28. Indeed, in our study, age ≥ 65 showed a trend to a reduced incidence of this adverse event. We hypothesized that already diminished bile acid production and excretion, as occurs in older patients 29, could have been an influence in our findings.
Parenteral nutrition liver disease (PNALD) and LFT alterations associated to PN are considered to be of multifactorial origin and have a decreased incidence in adults compared to infants. Main factors in its development are excess caloric intake of macronutrients, specially fats, infectious or septic events, inflammation, oxidative stress, nutrient deficiencies, and contaminants in parenteral products 30. In general, our findings are in agreement with this knowledge: surgery as a pro-inflammatory factor and increased fat load.
Nevertheless, amino acid load has been found to reduce its incidence in our study. This parameter has not been studied extensively in adults and usually has not been considered to play a role in PNALD currently 17. To our knowledge, only one prospective study found that a load > 1 g protein/kg/day was deleterious for liver parameters 31. The role of amino acid load in LFT alterations during PN would require further studies.
In PN, the contribution of concomitant medications to adverse events has not been assessed in depth. The simultaneous use of some medications during PN has been shown to be of relevance for developing hyperglycemia 15,21 or hypertriglyceridemia 16, but we could not find studies for LFT alterations or PNALD. In our work, the simultaneous use of two or more medications has been associated with a moderate contributory influence in LFT alterations and a strong influence in hyperglycemia. These results on the effect of medications in PN toxicity warrant further investigation.
This is the first multicenter study that has evaluated the metabolic complications of PN in patients of ≥ 65 years compared to younger patients, but our study had several limitations. They include its observational design, and differences in amount of nutrients administered. Regression models adjusted by some of these limitations have been used to manage them. Besides, observational cohort studies can provide a reflection of daily clinical practices. The groups had several differences that replicated the differences that would be expected to be found between younger and older patients. Older patients have usually more comorbidities, worse renal function, and lower albuminemia.
Nutritional loads were different between the two groups, but both received the same amount of energy intake in relation to their cREE. This approach would be acceptable in our opinion. In clinical trials, it has been acceptable to use different medication doses in older people to reach the same efficacy 32. Critically-ill patients were not included in order to have a homogenous population, as well as to have a controlled number of variables to be adjusted for. Therefore, the results obtained could not be extrapolated to this group of patients. Finally, other PN safety variables, such as catheter-related infections, acid-base disturbances, metabolic bone disease, and other alterations were not assessed. We assessed the most prevalent adverse effects related to PN in hospitalized patients, since for less prevalent events a larger cohort would be needed.
In conclusion, non-critically ill inpatients of ≥ 65 years receiving PN had the same incidence of hyperglycemia, hypertriglyceridemia, and LFT alterations as younger patients when administered a caloric intake adapted to their cREE. In addition, the older age group may have a trend to a lower prevalence of LFT alterations related to PN.