INTRODUCTION
Smoking, determined by nicotine dependence, is now considered by the World Health Organization (WHO) as the leading cause of preventable death in the world 1. Furthermore, it is a triggering factor for many diseases, among which some stand out, such as cardiovascular condition, cancer and obstructive pulmonary chronic disease 2.
Adipose tissues produce adiponectin and leptin to regulate eating behavior and also generate pro and anti-inflammatory behaviors of adipokines to modulate inflammatory responses 3. While adiponectin protects, the leptin accelerates the development of atherosclerosis. Therefore, it is suggested that these adipokines establish a close correlation between smoking and the appearance of cardiovascular and metabolic diseases 4.
The interaction of leptin with its receptor in the hypothalamus alters the release signals that affect appetite 5. Its action on the central nervous system inhibits food intake, increases energy expenditure and regulates the metabolism of glucose, fats and neuroendocrine function 6. In addition, leptin is involved in the recruitment process, activation and survival of inflammatory cells 7, and it has been considered as a potential mediator of weight gain after smoking cessation 8. However, studies on the association of smoking and leptin are still controversial, with reduced and increased reports, or no effect on it 9,10,11.
Adiponectin is associated with insulin sensitivity, a marker of metabolic syndrome 12. It acts in inhibiting proliferation of vascular smooth muscle cells, suppresses the conversion of macrophages into foam cells 13, and also acts in the reduction of mediators related to pro-inflammatory effects such as C-reactive protein, interleukin-6 and tumor necrosis factor 14. Studies conducted with smokers have pointed to a reduction in adiponectin levels in smokers 15,16.
Ghrelin is a peptide produced in the stomach with properties of energy balance and induction of appetite. It is known that fasting stimulates its activity mediated by hypothalamic neuropeptide Y and agouti-related protein system 17. According to previous studies, plasma ghrelin levels do not correlate with smoking years, but with the current smoking, indicating acute effects of smoking in the ghrelin concentrations 17,18.
Nicotine acutely increases energy expenditure and may reduce appetite, which probably explains lower body weight among smokers 19.
The weight gain evidenced in smoking cessation can be attributed to the reduction of the thermogenic effect caused by tobacco and to an increased food intake. These senses may be affected by continued cigarette use, favoring inadequate food choices, such as high caloric density and low nutritional quality foods, exacerbating weight gain 20. Attention should be given to excessive weight gain and the return of smoking, in order not to increase the risk of morbidity and mortality by endocrine-metabolic, cardiovascular, pulmonary, circulatory, gastrointestinal diseases and cancers 19,21.
Considering the many endocrine-metabolic changes involved in smoking which are not fully understood, the small number of studies that assessed leptin, ghrelin and adiponectin simultaneously in smoking cessation 22 and the impact of smoking mortality in the public health, it is important to know the anthropometric, biochemical and endocrine profiles of smokers in withdrawal process.
It is believed that smoking cessation may lead to reduced serum levels of inflammatory parameters such as leptin, LDL cholesterol and triglycerides, and improve the level of anti-inflammatory parameters such as adiponectin and HDL cholesterol. Therefore, the aim of this study was to evaluate serum concentrations of pro- and anti-inflammatory substances in smokers at baseline and after four months of treatment for smoking cessation.
METHODS
DELINEATION AND STUDY POPULATION
The present study was an intervention study conducted with a non-probabilistic sample, consisting of evaluated smokers attended at the Interdisciplinary Center for Research and Intervention on Tobacco, University Hospital, Federal University of Juiz de Fora (CIPIT/HU-UFJF), for the treatment in intensive approach in the period from September 2011 to February 2014.
The work by CIPIT included prevention activities, treatment and tobacco control, with a multidisciplinary approach based on the guidelines of the National Tobacco Control Program (NTCP) of the National Cancer Institute. Smokers received intensive smoking cessation counseling in structured weekly sessions for one month, followed by two biweekly and then monthly sessions for 12 months. For the maintenance of abstinence, the study participants used the Nicotine Replacement Therapy (NRT) as therapeutic scheme proposed by the doctor clinic.
INCLUSION AND EXCLUSION CRITERIA
Participants were men and women over 18 years of age who were diagnosed by physician-administered CIPIT HU/UFJF with smoking by the International Classification of Diseases, 10th edition, used tobacco as a principal drug and accepted to participate in the research by signature of the Free and Clarified Consent Term. The following patients were excluded from the study: patients under 18 years; pregnant women; patients with kidney disease, acquired immunodeficiency syndrome, liver disease, chronic obstructive pulmonary disease; and those who refused to participate. The proposal was approved by the Ethics in Research Committee of the Federal University of Juiz de Fora (CAAE: 0067.0.180.420-11/no. 081/2011). The study protocol conforms to the ethical guidelines of the National Health Council no. 466/2012.
SMOKING HISTORY
We accessed the smoking history of abstinent and smokers, including the amount of cigarettes smoked per day and the duration of smoking. The smoking index was available (number of cigarettes smoked per day × number of years that the smoker remains smoking) 23. The scores of the Fagerstrom test of nicotine dependence (FTND) to identify the behavior of smokers were also evaluated. The sum of the values indicates one score of the degree of dependence: low (0-4 points), moderate (5 points) or high (5-10 points) 24.
VARIABLES ANTHROPOMETRIC ANALYZED
Anthropometric assessment was performed at baseline, after one month, and at the end of the treatment (four months). Nutritional assessment was based on measurements of body weight and height to calculate body mass index (BMI). To measure body weight, Welmy(r) was used, with a capacity of 150 kg and precision of 0.1 kg, with the participants standing barefoot and wearing light clothing. The classification was performed as proposed by the World Health Organization 25. To measure the height, the portable anthropometric Alturaexata(r) was used.
Waist circumferences (WC) were measured in the mid-point between the lower end of the rib cage and the top of the iliac crest in a standing position 25.
The body adiposity index (BAI) was determined by measurements of hip circumference (HC) and height to access the body fat: BAI = (HC [cm]/height [m] √height [m]) - 18. The cut-off points used were 32 for women and 27 for men 26.
Waist-to-height ratio (WHtR) was determined by the ratio of (WC [cm]/height [cm]) and the cut-off point adopted for discrimination of central obesity and cardiovascular risk was ≥ 0.5 for both sexes 27.
The HOMA index, described by Matthews et al. 28, was evaluated by the equation HOMA-IR = fasting insulin (mU/l) x fasting blood glucose (mmol/l)/22.5.
BIOCHEMICAL ANALYSIS
Blood samples were collected after eight-hour fasting and centrifuged within 30 to 45 minutes of collection and stored at -80 °C until analysis. Laboratory tests were requested at the beginning and after four months of treatment by the CIPIT-HU/UFJF doctor and performed in the Clinical Analysis Laboratory at HU/UFJF. The cut-off points for the biochemical variables analyzed were: total cholesterol (140-200 mg/dl), LDL-c (100-129 mg/dl), HDL-c (40-60 mg/dl), triglycerides (65-150 mg/dl), glucose (70-99 mg/dl), cortisol (5-23 mcg/dl) and insulin (1.9-23 mg/dl).
ANALYSIS OF ADIPOKINES AND GHRELIN
The serum levels of leptin, adiponectin and ghrelin was determined by ELISA. The kits for measurement of adiponectin, leptin and ghrelin were produced by Millipore(r), USA. The sensitivity of detection of leptin were 0.78-100 ng/ml, 1.5-100 µg/ml for adiponectin and 50-5,000 pg/ml for ghrelin.
STATISTICAL ANALYSIS
For sample characterization, descriptive tables with measures of central tendency and dispersion of variables were used.
The Kolmogorov-Smirnov test was used for the assessment of the normality of data. Wilcoxon paired tests were used for the initial variables and after four months of treatment. Spearman's correlations were used to assess the association between anthropometric and biochemical variables, and serum levels of adipokines and ghrelin. The U-Man Whitney test was used to compare the variables between smokers and abstainers. Linear regression was used to adjust the concentration of leptin by BMI and WC The values of ghrelin and adiponectin did not need to be adjusted because they did not present statistical significance in the linear regression, only leptin. The significance of the model was evaluated by the F test of analysis of variance and the quality of it by the adjusted determination coefficient (adjusted R2). The residues were evaluated according to the suppositions of normality, zero mean, constant variance and independence, and all the assumptions were followed. The L/A ratio was calculated 29.
The data were processed and analyzed using SPSS 23.0 software and, for the purpose of interpretation, the limit type I error was up to 5% (p < 0.05).
RESULTS
After four months of treatment, among the 86 participants in the study, 29 completed the treatment for smoking cessation, including 22 women and seven men. Of those, 22 were abstinent and seven had some cigarette consumption. The mean age of participants was 50.7 ± 10.47 years. These 29 previous smokers have had a long duration of smoking (34.57 ± 12.21 years) and had smoked almost one pack/day (19.3 ± 7.98 cigarettes/day). The smoke index was 666.84 ± 396.37 (cigarettes/day)/year. The nicotine addiction was high for most of the group (40%), as confirmed by high mean of FTND score (5.20 ± 2.12). The variables did not have statistically significant differences when the values for the abstinent and smokers were compared (smoking duration, p = 0.85; cigarettes/day, p = 0.08; smoke index, p = 0.23; FTND score, p = 0.58).
When comparing smokers and abstinent, in those who completed treatment, they had similar values for all anthropometric (Table I) and biochemical (Table II) parameters evaluated at the beginning and end of the treatment. For BMI, both abstinent and smokers had overweight classification as average values. The BAI indicated excess of body adiposity for 100% of the patients, at the beginning and at the end of the treatment. WHtR was high in both groups at the beginning and end of treatment. However, the abstainers showed a reduction in mean values and median of WHtR (Table I).
Table I Comparison of the mean values of anthropometric parameters at the beginning and after four months of treatment for smoking cessation, according to smoking status

Note: A,B different capital letters on the same line and a,bdifferent lowercase letters in the same column for each type of variable indicate a statistically significant difference, adopting p < 0.05. BAI: Body adiposity Index; BMI: body Mass Index; WC: waist circumference; WHtR: ratio waist/height
The variation of anthropometric variables, such as weight, BMI, WC, BAI and WHtR, at the beginning and end of treatment were similar between smokers and abstinent (p > 0.05). However, the abstinent showed weight gain and abdominal fat accumulation in the period of four months, with increased weight p < 0.001, BMI p = 0.006, and WC p = 0.017 (Table I).
Comparing the initial and final values of biochemical parameters and insulin resistance, no statistically significant differences (p > 0.05) were found (Table II).
Table II Comparison of the mean values, median, minimum and maximum of biochemical parameters at the beginning and after four months of treatment for smoking cessation, according to smoking status
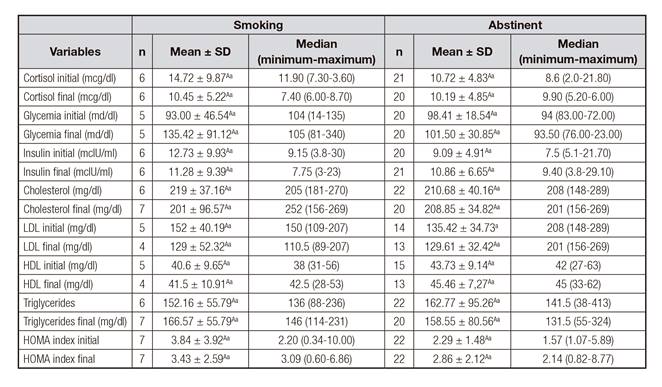
Note: A,Bdifferent capital letters on the same line and a,bdifferent lowercase letters in the same column for each type of variable indicate a statistically significant difference, adopting p < 0.05.
At the beginning of the treatment, all smokers had similar levels of adiponectin. At the end, the abstinent had higher levels of adiponectin compared with those who remained smokers (p = 0.024) (Table III). Regarding adipokines, there was an increase in the average of leptin/WC (abstinent p < 0.001; smokers p = 0.028) and a reduction in adiponectin levels after treatment, in both, abstinent (p = 0.048) and smokers (p = 0.043). The value of leptin/BMI initial was increased only among abstinent p = 0.003. The L/A index increased during the treatment for smokers (p = 0.028) and abstinent (p = 0.044) (Table III).
Table III Comparison of the mean values, median, minimum and maximum of cytokines at the beginning and after four months of treatment for smoking cessation, according to smoking status
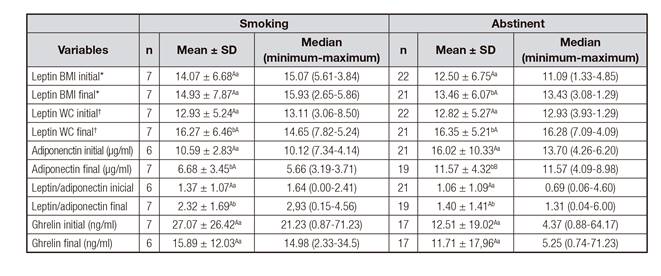
*Leptin adjusted by BMI. †Leptin adjusted by WC. Note: A,Bdifferent capital letters on the same line and a,bdifferent lowercase letters in the same column for each type of variable indicate a statistically significant difference, adopting p < 0.05.
Table IV shows the correlation between the initial and final levels of leptin adjusted for BMI and WC, leptin/adiponectin ratio, ghrelin and adiponectin with the initial and final anthropometric variables, according to the status of abstinent or smokers. Among those who continued smoking, there was a positive association between the initial leptin/BMI with WC, WHtR and BAI; initial leptin/WC with BMI, WHtR and BAI. There was a positive association between the final leptin/BMI with weight, WC and BAI; final leptin/WC with BMI, weight WHtR and BAI; and final L/A with BMI, weight and BAI.
Among the abstinent, there was a positive association between initial leptin/BMI with weight, WC, WHtR, BAI and HOMA-IR; initial leptin/WC with BMI, WHtR, weight, BAI and HOMA-IR; and initial L/A with BMI, WHtR and HOMA-IR. The variables with a significant correlation after four months among abstinent were leptin/BMI with weight, WC, WHtR, BAI and HOMA-IR; leptin/WC with BMI, weight, WHtR, BAI and HOMA-IR; and final L/A with BAI (Table IV). The ghrelin did not correlate with any of the analyzed variables (data not shown in table IV).
DISCUSSION
The results of this study showed that smokers who completed the treatment at the Interdisciplinary Center presented a high degree of dependence, cigarette consumption and long duration of smoking. These factors may make treatment difficult and precipitate relapse; however, the benefits of smoking cessation should be used as support: the risk of cardiovascular diseases is reduced by half when compared with those who continued smoking. Between five and 15 years post-smoking, the risk of stroke and coronary heart disease is similar to that of those who never smoked 30.
Smoking cessation has been shown as an improvement factor in adiponectin profile with different time periods in various studies (4, 9, 12 and 24 weeks) 4,22,32,33.
We could not find a significate difference in our study, most likely because of the small sample size. Nevertheless the abstinent group showed higher serum levels after four months of treatment. Some authors have identified a reduction in adiponectin levels in smokers 18. Nicotine, the main active component of cigarettes, may decrease adiponectin in part by altering the ATP-dependent potassium channels in adipocytes 31.
The increase in body weight among the abstinents may have attenuated the response of adiponectin at the end of the treatment, as it has been demonstrated that body weight reduction increases adiponectin levels in plasma. It has been suggested that adiponectin may be under rigorous regulation of feedback of body fat 34. In assessing abstinent participants who gained and maintained body weight at the end of the treatment 35, reduced adiponectin serum levels were found in the group gaining weight. Among abstinent whose body weight remained stable, the same was not observed. In this study, there was no significant correlation between adiponectin and final weight, BMI, BAI and WC in abstinent.
The sensitizing effect of insulin occurs through binding to the adipoR1 and adipoR2 receptors, leading to activation of AMP-activated protein kinase (AMPK), protein phosphorylation receptor-α activator (PPAR-α) and, supposedly, other signaling pathways still unknown. In insulin resistance associated with obesity, the adiponectin receptors and adiponectin are repressed 31. Unexpectedly, in this study, no significant association between adiponectin and HOMA-IR was found for smokers and abstinent at the beginning and the end of the treatment.
Various measures of insulin sensitivity are strongly dependent on the L/A ratio, emphasizing the role of adipocyte dysfunction in the pathogenesis of insulin resistance 36. In this study, the L/A ratio did not have a positive correlation with HOMA-IR in abstinent at the beginning of the treatment. In a different study population, Kappelle et al. (2012) 37 suggest that the leptin/adiponectin ratio is a preferential marker of atherosclerosis susceptibility, and a preferential marker of associated cardiovascular events with adipokines in men. At the end of the treatment, L/A ratio demonstrated a positive correlation with BAI values, reinforcing the role of adipocytes in the control of serum leptin levels.
The increase in L/A levels over the treatment for smokers and abstinent found in this study may suggest a risk of cardiovascular events; however, this relationship should be investigated in the smoking population in order to predict and monitor the risk of associated cardiovascular events to tobacco.
The impact of smoking cessation on serum leptin levels still generates controversial results 9,10,11. In this study, leptin/WC increased over the treatment for smokers and abstinent. The mechanisms involved in the changes in leptin levels after smoking cessation remain unknown.
Nicotine provides an increase in plasma cortisol concentration and can also promote the increase or decrease in leptin concentration. The results of our preliminary study showed an inverse relationship between leptin concentration and cortisol levels, indicating that higher concentrations of leptin are associated with lower levels of cortisol 16. However, the increase in leptin after treatment has not been accompanied by an increase in cortisol levels, regardless of the smoking status.
Other factors which have not been assessed in this study can directly influence the production of leptin, because the increase in plasma leptin levels occurred even after adjustment for body composition parameters as BMI and WC, as opposed to the findings by Nicklas et al. 38. The increase in body weight and leptin in this study supports the hypothesis that chronic inflammation induced by cigarette metabolites could damage the leptin receptors, thus not interfering in the appetite and reduced weight 39.
Plasma ghrelin levels decreased after smoking cessation, but this change was not significant, as reported by Won et al. 20. The effect of smoking on ghrelin levels appears to be acute 18. The plasma ghrelin levels during treatment did not correlate with any of the body composition parameters.
The weight gain after smoking cessation has been reported in the literature. Studies report that the average weight gain after smoking cessation may be 2.8 kg in women and 3.8 kg in men, able to come to gain 10 kg, in short or long-term 17. Another study showed that, on average, abstinent gained 2.2 kg in two months and continued to gain up to 4.5 kg in 12 months 40.
In this study, the mean weight gain during the four months of treatment was 1.23 kg for smokers and 1.86 kg for abstinent, accompanied by an increase in WC of 1.34 cm for smokers and 1.33 for abstinent. Despite weight gain and WC increase among abstinent patients, there was no significant increase in body fat indicated by BAI. The presence of the Nutrition staff in association with smoking cessation may have influenced the results, supporting volunteers in times of abstinence and anxiety by lack of cigarette, guiding the qualitative food choice. The weight gain in abstinent can be attributed to the influence of smoking in energy metabolism, with reduction of the metabolic rate and the improvement of taste. The perpetuation of some inadequate food choices established during smoking can aggravate weight gain due to energy imbalance, with high caloric density choices and low nutritional value 41. Despite being an intervention study with comparison of anthropometric, biochemical and adiponectin, leptin and ghrelin levels of smokers at the beginning and after four months of treatment for abstinence, it has some limitations. Firstly, the limited sample size due to the abandonment of volunteers throughout the treatment. A total of 159 subjects began treatment, whereas 86 were included in this study, and only 29 subjects remained until the end of the four-month duration treatment. Another limitation is the possible influence of physical activity, medications and genetic predisposition in the results of biochemical tests. In addition, the influence of physical activity on anthropometric results was not evaluated. We have shown that smoking cessation improves adiponectin levels when compared with smokers. The increase of leptin in the period of abstinence, independent of weight gain, still remains as a mechanism which has not been elucidated. The higher leptin/adiponectin rates at the end of the treatment may indicate a risk of cardiovascular events in patients receiving treatment.
The reduction of ghrelin, although not significant, strengthens the role of leptin on its control, since its levels increased throughout the treatment in both groups. Such evidence suggests an association among appetite controlling hormones (ghrelin and leptin) and adiponectin, and energy metabolism, weight change and body composition, which all play a role in body metabolism and fat distribution.















