INTRODUCTION
End-stage renal disease (ESRD), as well as hemodialysis (HD), treatment are marked by clinical-nutritional conditions that increase morbidity and mortality and reduce life quality of patients with this chronic disease 1,2,3. In this sense, hypoalbuminemia, which is also affected by inflammation status and age, is not able to accurately reflect the nutritional state 4. In turn, body mass index (BMI) has been associated with better prognosis of individuals in HD, which is known as "reverse epidemiology" 5,6,7. This relationship is influenced by characteristics such as age, inflammation and related-comorbidities 8. In addition, subjective global assessment may help predict mortality, when properly applied 9.
On the other hand, the progressive deterioration of renal function leads to physiological dysfunctions such as changes in cellular energy metabolism, protein catabolism, insulin resistance and synthesis of mediators of inflammation and oxidative stress 10,11,12. Still, the complex inter-relationship among nutritional indicators and inflammatory and oxidative markers remains the object of investigation on the premise to get better prediction of mortality in patients with ESRD in HD 13.
Overall, the objective of this integrative review was to present and discuss the latest scientific literature on the ability of clinical-nutrition indicators, and inflammatory and oxidative stress markers to predict morbidity and mortality in HD individuals.
STUDY CHARACTERISTICS AND SELECTION CRITERIA
A review search was carried out from the databases LILACS, Medline, PubMed, SciELO and BIREME, using the keywords "hemodialysis and mortality", "chronic renal failure", "ESRD", "biochemical markers", "inflammatory markers", "oxidative stress markers", "anthropometric evaluation", "nutritional status", "subjective evaluation", combined with " mortality". Publications carried out from 2010 to 2016 with HD individuals were included.
From the selected articles, a reverse search was carried out for studies whose titles would be eligible. Subsequently, the abstracts were read to ensure compliance with the inclusion criteria and then each article was entirely read to confirm its eligibility. Cohort studies with adults and elderly individuals in HD treatment were included. Articles that were not published in full or those presented as tutorials, editorials, news, letters or comments, reviews and experimental testing were excluded. In addition, studies of acute renal disease, chronic kidney disease under conservative treatment or treatment with peritoneal dialysis, transplantation and nephrotic syndrome were excluded.
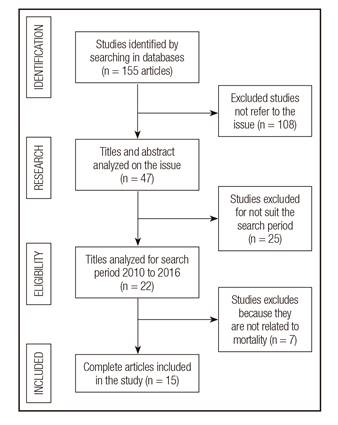
During the initial selection process, 155 articles were found, from which 108 were excluded, as shown by Figure 1. Selected papers are related to anthropometric indicators (four studies), subjective global assessment (five studies), oxidative stress markers (two studies), and inflammatory markers (four studies) as predictors of mortality in HD individuals.
CLINICAL-NUTRITIONAL PREDICTORS AND MARKERS OF INFLAMMATION AND OXIDATIVE STATE OF THE STUDY
The 15 selected studies used as anthropometric indicators, BMI, waist circumference (WC), skinfold thickness, arm circumference (AC), Mid-arm circumference (MAC), mid-arm muscle circumference (MAMC) lean tissue index (LTI) and fat tissue index (FTI) and total body fat (TBF), assessed by bioelectrical impedance analysis (BIA). Subjective methods to nutritional assessment were: Subjective Global Assessment (SGA), Modified Subjective Global Assessment (mGSA), Objective Score of Nutrition on Dialysis (OSND), International Society of Renal Nutrition and Metabolism (ISRNM), Malnutrition-Inflammation Score (MIS) and Geriatric Nutritional Risk Index (GNRI).
Hemoglobin, albumin, calcium, phosphorus, parathyroid hormone (PTH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total cholesterol (TC), triglycerides (TG), uric acid, urea, creatinine, Kt/V urea (Kt/V), ferritin and transferrin were used as metabolic markers.
The oxidative stress markers used in the studies were plasma concentrations of gamma-glutamyltransferase (GGT), nitric oxide (NO) and malondialdehyde (MDA); while the inflammatory markers were plasma concentrations of C-reactive protein (CRP), interleukin-6 (IL-6), tumor-necrosis factor alpha (TNF-) and theirs receptors (TNFR1 and TNFR 2).
The articles were separated in accordance with categories of predictors of mortality in HD as follows: anthropometric indicators, subjective scores, oxidative stress and inflammation markers (Table I, Table II, Table III, Table IV).
Table I Comparative studies of anthropometric predictors in the mortality of hemodialysis patients (cohort studies, 2010-2016)

AC: arm circumference; BMI: body mass index; BSF: bicipital skinfold; CRP: C-reactive protein; FTI: fat tissue indices; HD: hemodialysis; LTI: lean tissue indices; MAC: mid-arm circumference; MAMC: mid-arm muscle circumference; TSF: triceps skinfold; SSF: subscapular skinfold.
Table II Comparative studies of subjective predictors in the mortality of hemodialysis patients (cohort studies, 2010-2016)

AC: arm circumference; BMI: body mass index; HD: hemodialysis; ISRNM: International Society of Nutrition and Metabolism Renal; MAC: mid-arm circumference; MIS: malnutrition-inflammation score; OSND: Objective Score of Nutrition on Dialysis; SGA: subjective global assessment; TSF: triceps skinfold.
Table III Comparative studies of oxidative stress predictors in the mortality of hemodialysis patients (cohort studies, 2010-2016)

ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; CRP: C-reactive protein; Cca: corrected calcium/albumin; GGT: gamma-glutamyltransferase; HD: hemodialysis; MDA: malondialdehyde; PC: protein carbonyls; iPTH: intact parathyroid hormone.
Table IV Comparative studies of inflammatory predictors in the mortality of hemodialysis patients (cohort studies, 2010-2016)

BMI: body mass index; CRP: C-reactive protein; GNRI: geriatric nutritional risk index; HD: hemodialysis; IL-6: interleukin-6; TNF-: tumor necrosis factor-alfa; sTNFR1 and 2: tumor necrosis factor receptors 1 and 2; VFA: visceral fat area; SFA: subcutaneous fat area; SGA: Subjective Global Assessment.
ANTHROPOMETRIC INDICATORS ON MORTALITY IN HD INDIVIDUALS
Studies show that protein-energy malnutrition is present in a range of 23% to 76% of individuals in HD 14. Unlike the general population, a higher BMI in these individuals was associated with better survival, fact presented as reverse epidemiology in the literature 15. However, BMI measures did not present differences between lean and fat mass, making it difficult to quantitatively understand which components of body composition are related to survival in HD individuals with ESRD 16. In order to evaluate body compartments, the studies use the Body Composition Monitor (BCM) based on spectroscopic bioimpedance (BIS). Bioimpedance methodologies, the BCM expresses body composition as a three-compartment model, providing overhydration, lean tissue index (LTI), and fat tissue index (FTI), whereby LTI and FTI are the respective tissue masses normalized to height squared. Also, LTI and FTI percentiles (< 10th percentile [low]; 10th-90th percentile [normal]; and > 90th percentile [high]) relative to an age- and sex-matched healthy population are supplied. The three-compartment model of the BCM has been validated against standard reference methods for assessment of fluid status and body composition in dialysis patients. Castelhano et al. 17 have indicated LTI and FTI reference values (10th and 90th percentiles), adjusted for age and sex, in which lean tissue below the 10th percentile was associated with higher mortality in HD (OR: 1.57). Similarly, considering age, sex and diabetes mellitus, higher percentage of lean body mass was associated with better survival 18,19 in HD individuals for the same population.
Moreover, Rosenbergeret et al. 20 evaluated the relationship between scarcity of lean tissue (expressed as LTI below the 10th percentile) and survival in HD. The possible causes to worse survival 21,22 included malnutrition and inflammation signal 21 as lean mass stocks uremic toxins, in which case, the smaller amount of lean tissue could indicate a higher concentration of uremic toxins in the blood 21,23.
The relation of muscle mass and increased mortality caused by infections in HD individuals is well known, since subjects with ESRD develop an acquired immune deficiency that may be exacerbated in those with low muscle mass and hipoalbuminemia 24. In this sense, Marcellid et al. 16 had found that body composition by multifrequency bioimpedance and LTI and FTI between the 10th and 90th percentiles were associated with improved survival, while the low LTI and FTI, and especially the combination of both, were associated with increased mortality, regardless of BMI.
Other anthropometric and body composition indicators do not qualify as common practices. TBF and simple anthropometric measures, such as MAC, AC and triceps skinfold thickness (TSF) are generally used 25,26. Stosovic et al. 27 found that the TBF, TSF, AC and MAC were independent predictors of mortality for individuals in HD. The predictive values of all these anthropometric indicators for mortality were similar, except for BMI. When these indicators were altogether tested, the AC was the indicator with the greatest power to predict mortality and showed an average reduction of 8.0% in the proportional mortality risk.
Furthermore, the reduction of muscle mass in this population 28 is associated with hypoalbuminemia and PEM, which indicate inflammatory conditions 29. Su et al. 24 have reported that the decline in lean body mass over time, estimated by MAC and skinfold measurements were associated with higher risk of all specific causes of mortality in HD individuals. These relationships were particularly strong in those with BMI < 25 kg/m2.
It is noteworthy that BMI values for individuals in HD are usually higher than for general population, although many of them may present LTI values below percentile 10 30, suggesting that body composition related to lean tissue is more important than BMI isolated. In addition, interventions to keep lean and fat mass suitable are favorable for survival in population with ESRD. Thus, AC measure and the body fat distribution (as LTI and FTI) were altogether promissory predictors of morbidity and mortality in HD, reinforcing the importance of lean and fatty tissues in evaluating the survival of HD patients independently of BMI, making them (mainly AC) good indicators in clinical practice.
SUBJECTIVE SCORES ON MORTALITY IN HD INDIVIDUALS
In this sense, SGA is a simple method for assessing the nutritional status in many patients, including those with ESRD. Vogt and Caramori 31 had presented prevalence of malnutrition of 26.3% by SGA, 25.2% for MIS (MIS > 8) and 28.8% by criteria based on ISRNM, and malnutrition evaluated by SGA and MIS was able to predict mortality in a period of 15.5 ± 5.4 months. The study of Zuijdewijnet et al. 32 highlighted the SGA and MIS, after comparing eight evaluation tests of nutritional risk, as better predictors of mortality. As results, these studies show the impact of changes in scores of SGA and MIS on clinical outcomes and mortality risk. Another study has shown that an 1-point increase in SGA, 12 months after dialysis, was independently associated with an increase of 34% in all-cause mortality risk when tested with albumin, CRP, and BMI 33. The same finding was discussed by Chan et al. 34, after adjusting for all variables including age, sex, HD time, serum albumin, body mass index and smoking. In addition, they observed that the SGA, considering mild malnutrition (B) and moderate (C) independently predicts mortality.
The study of Beberashvili et al. 35 has found significant associations with hospitalization and mortality in individuals in HD through a comprehensive OSND scoring system. Both variables were significantly correlated with inpatient days and frequency, as well as lean and fat body mass, MIS, blood pressure and IL-6 values 35,36. The results found by Beberashvili et al. 37 indicate that MIS had reliability and good concurrent and predictive validity 37.
Even so, these scoring systems should be considered as complementary clinical markers of malnutrition state, while application of SGA and MIS could result in early detection of malnutrition compared to metabolic and inflammatory markers, or classic anthropometric indicators 38. Therefore, SGA and MIS may represent good tools for application in clinical practice, as they may contribute to an early identification of malnutrition.
OXIDATIVE STRESS MARKERS ON MORTALITY IN HD
The presence of oxidative stress, as well as anthropometric and subjective evaluation, has been related to the increase in morbidity and mortality in cardiac surgical patients, critically ill and renal patients requiring HD 39,40.
In HD individuals, increased oxidative stress results from an imbalance between pro-oxidant activity and anti-oxidant systems, more intensely, contributing to increased morbidity and mortality 41,42. Diabetes mellitus, advanced age, inflammation, excess of uremic toxins, bio-incompatibility of dialysis membranes 43 and intravenous iron therapy 44 are the main causes of increased pro-oxidant activity in these individuals 42.
Excess of oxygen reactive species (ROS) can produce cellular damage, interacting with biomolecules (proteins, lipids and nucleic acids) and, thus, have negative effects on tissue function and structure. ROS can react with polyunsaturated fatty acids that produce lipid hydro peroxides. MDA, a linolenic acid product of decomposition of the main final oxidation reactions of polyunsaturated fatty acids, is a useful indicator to evaluate oxidative damage 45,46. MDA can still interact with DNA and proteins, and can lead to mutagenic and cytotoxic effects and, possibly, is involved in the pathogenesis of various diseases, such as atherosclerosis 47). Low MDA values, which suggest a lower intensity of oxidative stress, are associated with better survival 45.
Rusu et al. 48 observed that the increase in MDA is associated with a higher ratio of albumin corrected calcium (cCA). It is known that elevated cCA is a predictor of mortality, since hypercalcemia and increased ROS can act synergistically in aggravating the severe vascular lesions found in HD individuals. MDA has a high predictive value for the mortality of these individuals and is related to the survival of this population, especially when associated with cardiovascular diseases.
GGT 49, a recognized biomarker for liver disease, was another marker of oxidative stress presented as a predictor of mortality in HD individuals. It is an enzyme with important role in the extracellular catabolism of glutathione, a representative intracellular antioxidant 50. GGT-mediated oxidative stress may be involved in the formation of coronary atherosclerotic plaques and endothelial dysfunction 51,52,53.
In this sense, oxidative stress may play an important role in the pathogenesis of atherosclerosis and cardiovascular risk in ESRD, thus contributing to the increase in mortality. More studies are necessary to identify potential biomarkers in these people. Still, an early evaluation through GGT and MDA may contribute to a better monitoring of oxidative stress presence, which is common among individuals with chronic kidney disease.
INFLAMMATORY MARKERS ON MORTALITY IN HD INDIVIDUALS
Inflammation has recently been recognized as an essential component in ESRD in HD, playing a unique role in its pathophysiology and is responsible, in part, by CVD mortality end all other causes 54. Moreover, inflammation is related to the development of PEM and other comorbidities. In fact, the increase in release or activation of pro-inflammatory cytokines such as IL-6 or TNF-, as well as acute phase protein CRP, may suppress appetite, cause muscle proteolysis and hypoalbuminemia 55. Furthermore, PEM and inflammation contribute independently to hypoalbuminemia and thus increase the risk for mortality in ESRD in HD 56.
Inflammation is often present in individuals in HD, and the use of central venous catheter has been linked to chronic inflammatory state 57. Faria et al. 58 have found that its use as vascular access for HD procedure was independently associated with mortality in patients with high concentrations of CRP and low triglycerides.
In this context, the inflammation as assessed by CRP is present between 30% and 60% of American north and Europeans individuals in dialysis 59. In addition, values of CRP higher than 5 mg/L 60 or 10 mg/L 61,62 have been positively associated to cardiovascular mortality. Inflammatory markers such as TNF- and CRP are powerful independent predictors of risk for atherosclerosis, cardiovascular disease and mortality in HD individuals 63. The study of Nakagawa et al. 64 has shown that TNF- and CRP were positively associated with the causes of cardiovascular mortality, after adjusting for age and sex. When stratified by GNRI, TNF- and CRP were positively associated with all-cause mortality, only in malnourished individuals. This is supported by the finding of Carlsson et al. 65, concerning the slightly higher values of TNFR 1 and 2 in subjects with malnutrition. However, inflammation can elevate risk of mortality in patients with ESRD in HD by increasing cardiovascular risk and malnutrition.
Thus, the inflammation in individuals in HD, particularly evaluated by the CRP, is not only related to the cardiovascular alterations, including atherosclerosis, but it is also one of the key points in the development of PEM, stimulated by the oxidative stress. This fact can be reversed through a better follow-up of these individuals through PCR, TNF-, IL-6, identifying the evolution of inflammation and providing better nutritional support, aiming to improve the clinical picture of the individual with ESRD in HD.
CONCLUSIONS
In the absence of a gold standard to assess the mortality risk of HD individuals, application of one of the subjective methods together with adiposity and lean mass indicators, and CRP concentration in the clinical-nutritional practice could offer more accurate mortality risk in this population. Although oxidative stress biomarkers in ESRD are important, more studies are necessary to identify a recognized oxidative stress marker as a mortality predictor in HD.