INTRODUCTION
Heart failure (HF) is a progressive syndrome with high morbidity and mortality, characterized by cardiac dysfunction, which causes inadequate blood supply to meet metabolic and tissue needs, and presenting survival rates and losses in years of life which are equally as poor as those associated with cancer 1,2. Cardiac cachexia is a grave condition that affects patients with HF, involving a metabolic and neuroendocrine syndrome marked by an exacerbated inflammatory state 3.
Various definitions are proposed for cardiac cachexia, resulting in a prevalence which varies from 10 to 60%, according to the diagnostic criterion used and population evaluated 4,5,6. Patients diagnosed with cardiac cachexia present a mortality rate of 50% in 18 months, with these high mortality rates associated with cachexiaindependent of other variables, such as functional classification (FC), sodium levels, VO2 (volume of oxygen) peak, and age 3.
In light of the negative impact of cachexia on patient prognosis and the lack of a universal definition for diagnosing it, in 2006 a consensus was elaborated by the Cachexia Society (Washington, DC) for diagnosing cachexia, based on the main characteristics of this condition, including criteria which address biochemical and nutritional alterations that reflect the complex physiology of this syndrome 7. The Cachexia Consensus allows clearer diagnosis to be carried out, indentifying alterations in the components of this syndrome, and differentiating cachexia from other conditions, such as sarcopenia, malnutrition without inflammation, and anorexia. This consensus has not been applied sufficiently yet, and until now only one study has been identified which adopted the proposed methodology 8. Thus, it is possible that the magnitude of cachexia has not yet been sufficiently described, and more studies are needed to estimate its prevalence and describe the nutritional and biochemical alterations in patients with cachexia, since most definitions use only weight loss to diagnose it.
With the previous criterion, which uses only weight loss to diagnose cachexia, it was not possible to carry out a clear diagnosis and distinguish cachectic patients from other less serious conditions. Therefore, it is possible that cachexia is not adequately diagnosed, resulting in a lack of treatment and bad prognosis for these patients. In this context, the aim of this study was to evaluate cachexia prevalence in hospitalized HF patients according to the Cachexia Consensus criteria, comparing it with another diagnostic method for cachexia, in order to evaluate the factors associated with it and analyze alterations in the components involved in diagnosing it.
METHODS
A cross-sectional study was carried out at a public hospital of reference in Cardiology, located in the Brazilian Northeast, and involving adult and elderly patients of both sexes diagnosed with HF and admitted between April and August 2015. Patients aged < 18 were excluded, along with those in the post-operative phase immediately after cardiac surgery; those with ascites, anasarca, upper or lower limb amputations; pregnant women; patients with a reported diagnosis of hepatic disease, hypo or hyperthyroidism or neoplasm; those living with acquired immunodeficiency virus (HIV); and those with renal disease in dialysis treatment.
The sample calculation was carried out considering 350 admissions occurring last year between April and August, with a 16.4% prevalence 5, 5% precision, and 95% confidence interval (CI), resulting in a minimum number of 131 patients to be studied. To correct for potential losses, this number was increased by 20%, bringing the total to 158 patients.
To diagnose cachexia, the criteria proposed by the Cachexia Society Consensus 7 were used, and for comparative purposes the diagnostic criterion proposed by Anker et al. (2003) was also used, which considers ≥ 6% weight loss in at least six months as a cachexia diagnosis 9. The Cachexia Consensus establishes a set of diagnostic criteria for cachexia, the main component of which is ≥ 5% non-edematous weight loss in the previous 12 months, or a body mass index (BMI) of < 20 kg/m2 when this weight loss cannot be documented, which should be combined with at least three characteristic markers of cachexia: loss in muscle strength, fatigue, anorexia, low muscle mass index, and/or biochemical alterations, such as inflammation, anemia, or hypoalbuminemia. Weight loss history was obtained in interviews with the patients, and the weight loss percentage was obtained by subtracting usual weight reported by the patients from the weight obtained at the time of anthropometric evaluations for the study.
The anthropometric measurements and application of questionnaires regarding fatigue, anorexia, and demographic data was carried out by two nutritionists trained for the study and using those patients admitted to the hospital over the research period and who met the study criteria.
Reduced muscle strength was evaluated using handgrip strength (HGS), which was measured with the help of a Jamar brand digital dynamometer, adopting kilograms (kg) as the measuring unit. The technique adopted follows the American Society of Hand Therapists' instructions 10. To classify HGS as low, the lower third for age and sex was considered 7. The fatigue evaluation was carried out using the Dutch Fatigue Scale (DUFS) 11, considering that a total score of ≥ 14.5 indicates the presence of fatigue. The anorexia evaluation was carried out using the simplified nutritional questionnaire on appetite (SNQA), and indices of ≤ 14 indicate risk of weight loss and the presence of anorexia 12.
Muscle mass was evaluated using the mid arm muscle circumference (MAMC). MAMC was obtained based on the mid arm circumference (MAC) and triceps skinfold (TSF) values taken from the non-dominant arm. The MAMC results were compared with the Frisancho (1981) standard for age groups up to 59 years old 13, and the Third National Health and Nutrition Examination Survey-NHANES III (1988-1994) standard was considered for the ≥ 60 age group 14. Muscle mass was considered to be low when MAMC was below the percentile 10 for age and sex.
Among the biochemical alterations for cachexia, the following were considered: anemia (hemoglobin < 12 g/dl), hypoalbuminemia, when albumin < 3.2 g/dl, and an inflammatory state, evaluated using the C-reactive protein (CRP ≥ 5.0 mg/l) 7.
The data regarding patient clinical variables were collected from the patient records: HF based illness, HF functional class (defined according to the New York Heart Association criteria), existing comorbidities (systemic arterial hypertension, diabetes mellitus, chronic rheumatic disease, and chronic renal disease), and the ejection fraction percentage of the left ventricle (%EFLV) (obtained from a cardiogram carried out during admittance). A %EFLV of < 50% was considered as being reduced. Nutritional state was evaluated using BMI, with adults classified according to the World Health Organization proposal (1997) 15 and the elderly classified using the cut-off point proposed by Lipschitz (1994) 16. Corrected mid arm muscle area (cMAMA) and mid arm fat area (MAFA) were evaluated, in accordance with the Frisancho equation (1990) 17.
The study was approved by the Committee on Ethics in Research involving human beings of the HUOC/PROCAPE hospital complex, in accordance with Resolution No. 466/2012 of the National Health Council/Health Ministry, under protocol number 980.472/2015. All of the patients and those responsible who agreed to participate in the study signed an informed consent form (ICF).
The data were tabulated and analyzed with the help of the SPSS statistical package, version 13.0 (SPSS Inc., Chicago, IL, USA). A descriptive analysis of the variables was carried out by calculating the frequency distributions and central tendency measures. The continuous variables were tested according to distribution normality using the Kolmogorov-Smirnov test. When they presented normal distribution, they were described in means and standard deviations and the respective parametric test was applied (Student's t test for comparing two means). When they presented abnormal distribution, they were described in medians and interquartile intervals, applying the non-parametric test (Mann-Whitney U test for comparing two medians). The factors associated with cardiac cachexia were analyzed using the Pearson's Chi-squared test or Fischer's exact test. To evaluate agreement between two diagnostic criteria for cachexia, the kappa index was used. The level of significance adopted for all of the tests was less than 0.05.
RESULTS
Of the 322 patients admitted during the period the study was carried out, 150 patients did not meet the eligibility criteria: edema (38), ascites 6, limb amputation 5, bed ridden patients for whom it was not possible to carry out the proposed evaluations 12, renal disease in dialysis treatment 18, HIV (4), cancer 3, post-surgery 17, endocarditis and other types of infection 29, mental disability 13, hypothyroidism 2, and hepatic disease 3. After eliminating losses from early hospital release 13, or inconsistent data 3, 156 individuals were included in the study, with an average age of 59.1 (± 15.3) and homogeneous distribution between sexes (Table I).
Among the patients with a HF diagnosis, the most prevalent functional class was functional class III (FCIII) (41.6%). A high percentage of patients with ejection fractions lower than 50% (51%) was verified. The most prevalent comorbidity in the sample was arterial hypertension (79.4%) (Table I).
Table I. General characteristics of hospitalized patients with heart failure, Northeastern Brazil (n = 156)

NYHA: New York Heart Association functional class; LVEF: left ventricular ejection fraction. *Chronic renal failure no dialysis.
Using the Cachexia Society Consensus criterion 7, 37.2% cachexia prevalence was verified, whereas the prevalence according to the Anker et al. criterion 8 was 49.3% (kappa = 0.401). In accordance with the Consensus criteria, the alterations in each parameter were analyzed, with fatigue and anorexia being the most prevalent aspects (88.2% and 72.1%, respectively). A ≥ 5% weight loss in the previous 12 months was observed in 61.7% of the sample, with low muscle mass found in 46.7% of the patients. PCR, an inflammation marker also used as a diagnostic criterion, was altered in 50.7% of the patients (Table II).
Table II. Prevalence of cardiac cachexia changes the proposed diagnostic criteria for Consensus in hospitalized patients with heart failure, Northeastern Brazil (n = 156)

CRP: C-reactive protein. *Low muscle strength: lower tertile for sex and age. †Score ≥ 14, assessed according to the Dutch Fatigue Scale (DUFS). ‡Score ≤ 14, assessed by Simplified Nutritional Appetite Questionnaire (SNAQ). §Assessed by arm muscle circumference. < percentile 10 for sex and age.
When the factors associated with cachexia were analyzed (Table III), there were no significant differences in prevalence in relation to age, sex, functional class, and comorbidities. Cachexia was most prevalent among the individuals with low BMI (p < 0.001), individuals with low muscle mass (p < 0.001), among the patients with an EFLV under 50% (p = 0.005), with low HGS (p < 0.001), with hypoalbuminemia (p = 0.040), and with anemia (p = 0.002).
Table III. Comparative analysis of variables associated with cardiac cachexia in hospitalized patients with heart failure, Northeastern Brazil (n = 156)
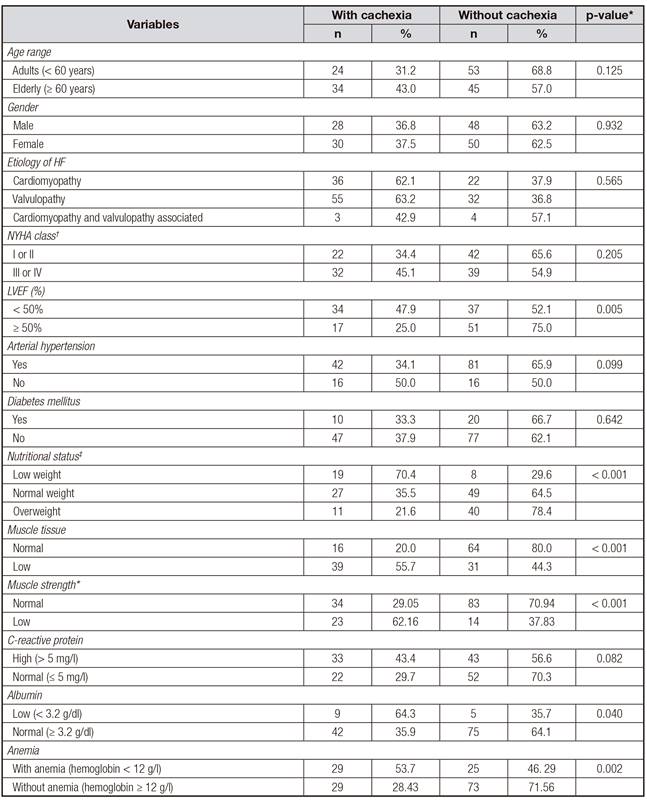
LVEF(%): left ventricular ejection fraction. *The Chi-squared test or Fisher's exact. †NYHA: New York Heart Association functional class. ‡Nutritional status according to body mass index (OMS, 1997). Muscle tissue assessed by arm muscle circumference. Muscle strength assessed by handgrip strength (kg), considering the lowest tertile for sex and age.
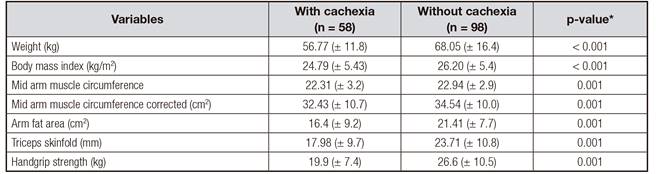
Lower averages were verified among the cachectic patients for weight (p < 0.001), BMI (p < 0.001), mid arm muscle circumference (p = 0.001), mid arm muscle circumference corrected (p < 0.001), arm fat area (p = 0.001), triceps skinfold (p = 0.001), and handgrip strength (p = 0.001) (Table IV).
DISCUSSION
The clinical symptoms commonly observed in HF, such as fatigue, dyspnea, and anorexia, associated with hypermetabolism and greater loss in nutrients, represent important factors which culminate in accentuated weight loss among these patients, and are responsible for a deteriorating nutritional state until serous conditions appear such as cachexia, which is an important predictor of poor clinical prognosis.
Cachexia is a syndrome that can affect HF patients and must be evaluated systematically during clinical monitoring of these individuals. There are various definitions and methods for diagnosing cachexia, resulting in differences in prevalences found. In our study, we used the most recent definition proposed by the Cachexia Society Consensus 7, and a high prevalence of 37.2% was found. Considering its repercussions in terms of a worse clinical evolution, reduced survival rate, and impact on hospital admittance numbers and costs, this high prevalence indicates a need for more attention to be paid by the whole multi-professional healthcare team. Previous studies that used only weight loss in the previous six months as the diagnostic criterion, and adopted different percentages (≥ 5%, ≥ 7.5%, > 6.0%), found cardiac cachexia prevalences which varied between 10.5%, 40.4%, and 19.0%, respectively 4,18,19. Letilovic and Vrhovac (2013), using the same consensus criteria adopted in this investigation in a sample of hospitalized HF and cancer patients, found a 21.8% prevalence of cachexia 8.
For comparative purposes, a previous definition was also used which involved only weight loss (≥ 6% in at least six months), identifying a higher cachexia prevalence (49.3%) than when the criteria proposed by the consensus was used (37.2%), and moderate agreement between the methods (kappa = 0.401). These differences in the prevalence found could possibly be attributed to the fact that the patients with weight loss only presented other conditions which are easily confused with cachexia, such as malnutrition without inflammation, malnutrition related with social and psychological conditions, and sarcopenia.
The findings in this study show ≥ 5% weight loss in 61.7% of the sample, which is higher than in the data presented by Letilovic and Vrhovac (2013), who evaluated hospitalized patients 8 diagnosed with cancer and HF (30.6%) and also higher than in the study with stable HF patients seen as outpatients, which showed ≥ 5% prevalence of weight loss in 42% of the sample 9. It is possible that the high prevalence of weight loss found in this study is influenced by the sample having been composed of patients admitted to hospital in a developing country with low social conditions and healthcare.
Weight loss in HF is considered to be the most sensitive indicator of clinical changes in cachectic patients, especially in patients with cardiac cachexia for whom weight loss mainly affects muscle, including cardiac muscle 20. Various factors can contribute to weight loss in HF patients, such as anorexia, poor nutrient absorption, dyspnea, and increased inflammatory cytokines 21. Although weight loss is the most important component of cachexia diagnosis, we believe isolated weight loss does not reflect the complex physiology of this syndrome. Moreover, weight loss evaluations with HF should be carried out with caution, given that these patients evolve with edema, and weight variations resulting from hydric retention are common, and thus nutritional evaluations should be carried out after edema resolution. Another aspect to be highlighted is that isolated weight loss does not differentiate cachexia from other conditions which also involve weight loss, such as anorexia, sarcopenia, and malnutrition without inflammation 22. Thus, we believe that the current consensus better represents cardiac cachexia due to it providing a systemic evaluation of this condition.
Studies show that despite cachectic patients presenting generalized loss of adipose and osseous tissue, muscle mass is compromised most, notably affecting the skeletal muscle 23. In our study, muscle loss was observed in 46.7% of the sample and 55.7% of the cachectic patients, which is a higher result than that reported by Fulster et al. 24 in a study with HF patients (19.5%) using DEXA (dual energy X-ray absorptiometry) to evaluate muscle mass, which is considered as the gold standard, instead of CBM, which is adopted in our investigation.
Involuntary weight loss plays a relevant part in compromising lean mass, given that it has been demonstrated that in some situations of unintentional loss in body weight, such as hypercatabolic clinical conditions, there is a disproportionate reduction in body stocks, originating an excessive loss of muscle tissue, which can be up to 72%, and a reduction of only 28% in adipose tissue, unlike with intentional weight loss, in which a reduction of 80% in fat mass and only 20% in lean mass are observed 25. The potential mechanisms involved in the preferential loss of muscle tissue result from excessive production of inflammatory cytokines, characterizing the exacerbated inflammatory state of cachexia, as well as increased catabolic hormone production, creating a disequilibrium between anabolic and catabolic processes. Cytokines have a direct negative effect on muscle mass, resulting in ingestion suppression, and the increase in catabolic hormones activates the ubiquitin proteasome pathway, which is the predominant pathway for protein degradation 23.
Although in our study PCR was not significantly associated with cachexia, it is known that this protein is not a sensitive and specific marker of inflammatory states, and for this reason the use of PCR was a limitation of our study. We suggest that another specific marker for evaluating cachexia inflammatory states, such as TNFα, would be more appropriate for evaluating this condition in future studies.
Fatigue is one of the most frequent symptoms of HF, especially in patients with functional class III and IV, involving symptoms of tiredness, exhaustion, and lack of energy, and associated with limitations in maintaining a lifestyle compatible with a desired sense of autonomy and independence, and is a limiting factor for physical activity among patients 26. Fatigue is one of the two most common symptoms (together with dyspnea) reported by HF patients 26,27, thus our results, which indicate a high percentage of this symptom (88.2%), were expected. The physiopathological causes of fatigue in HF are multifactorial 27 and include low cardiac debit, poor tissue perfusion, metabolic muscular abnormalities, abnormalities in the autonomous nervous system, physical deconditioning, and endothelial dysfunction.
Anorexia, present in 72.1% of the sample, may be related to HF via a connection with its main symptoms (fatigue and dyspnea). Anorexia can be a symptom of cachexia, however, it is a symptom that should be analyzed carefully, given that it also occurs in other conditions that are not associated with cachexia, such as the use of certain medications, depression, advanced ageing, and gastrointestinal problems. Moreover, anorexia can be due to a collateral effect of digitalis, angiotensin conversion enzyme inhibitors, intestinal edema, and sodium restricted diets 28,22.
Handgrip strength (HGS) is a fragility marker, is correlated with global muscle strength, and is considered as an independent predictor of a poor prognosis for HF patients. We identified low HGS in cachectic patients, with an average of 19.9 (± 7.4) kg, which is lower than in the data presented in a study of Japanese men 29 with HF (33.3 ± 8.6 kg), and lower than in the results from another investigation 30 involving patients with advanced HF (381 ± 7.9 kg). One previous study suggested a HGS value lower than 32.2 kg in HF patients as a predictor of mortality. As patients with cardiac cachexia present global myopathy, this would be expressed in an evident decrease in HGS, contributing to lower functional capacity, greater severity HF, and fatigue. The underlying mechanisms in HGS reduction include alterations in the ultra-structure and biochemistry of the skeletal muscle 30.
Anemia is a common condition in HF patients, causing a reduction in oxygen supply to the periphery, contributing to intolerance to exercise, and associated with greater clinical severity, rapid HF deterioration, and increased mortality. In our study, we found that anemia was significantly greater in cachectics (53.7%), and this result agrees with previous studies that demonstrate a high prevalence of anemia in HF patients, which is greater the worse the gravity of NYHA functional class, varying from 7.0% to 9.1% for FCI, and 65.9-79.1% for FCIV 31,32. In cachectic patients, some studies which evaluated anemia identified a prevalence which varied from 24% to 70% 18, independently of functional class. In cardiac cachexia, an increase in TNF occurs as a result of the inflammatory state of this condition, with this cytokine involved in bone marrow depression, induced erythropoietin insensitivity, and interference in iron release and use, and it is also known that inflammation reduces these patients' appetite, resulting in low iron ingestion 32.
Hypoalbuminemia is a common finding in cardiac cachexia patients, with prevalences varying from 18% to 89% (34). In our study, the cachectic patients presented significantly lower levels of albumin (64.3%), and the possible cause factors for this condition include malnutrition and systemic inflammation, which causes an increase in PCR levels. Other factors that explain hypoalbuminemia in these patients and that were not evaluated would be: intestinal losses caused by splenic congestion, renal insufficiency, and protein catabolism (34). The importance of evaluating albumin levels in these patients is due to its importance as an independent predictor of poor prognosis: it causes pulmonary congestion, results in greater oxidative stress and inflammation, favors edema of the myocardium, and subsequently aggravates myocardial dysfunction, contributing to fluid retention.
In our study, reduced %EFLV was significantly associated with cardiac cachexia. On the other hand, NYHA functional class did not present any relationship. Some studies demonstrate that reduced %EFLV and higher functional class (III-IV) are related with HF gravity and the occurrence of cardiac cachexia, and both have been used in evaluating HF prognosis, as independent mortality markers 18. Reduced %EFLV results in low cardiac debit, resulting in impaired peripheral blood flow. Hypoflux in cardiac cachexia has been shown to be an independent predictor of lower exercise capacity, loss of muscle strength, and an earlier onset of fatigue. In particular, hypoflux in the muscular skeleton causes ischemia of the tissue and hypoxia, causing significant alterations in the skeletal muscle via endothelial dysfunction, damaged vascular perfusion, the development of resistance to insulin, cellular death, and loss of muscle mass, causing weakening of the skeletal muscle. Moreover, hypoxia resulting from hypoflux is a determining factor for the pathogenesis of the metabolic alterations present in cardiac cachexia. In hypoxia, oxygen reduction causes a deviation of the metabolism to anaerobic pathways, resulting in increased mobilization of fatty acids, anaerobic glycolysis, and neoglucogenesis, causing increased catabolism (proteolisis) and reduced anabolism. In addition to these factors, hypoxia is considered to be the main stimulus for increased TNF production in HF patients. Therefore, hypoxia leads to a hypercatabolic and inflammatory state, which characterize cachexia síndrome (35).
Although cachexia was obviously greater among the malnourished, it is important to highlight that 35.5% of normal weight individuals and 21.6% of overweight individuals presented cachexia, showing that this condition is not restricted to low BMI cases. Not evaluating the cachexia criteria merely because patients have an apparently preserved nutritional state will hamper tracking individuals with this syndrome. Most of the time cachexia is only diagnosed and receives due importance when the patient reaches a devastating state of nutritional impairment. However, if patients are not systematically evaluated, their final evolution will be the characteristic underweight profile associated with cachexia.
In this study we identified the nutritional alterations in cachectic patients, and the factors which are associated with the occurrence of cachexia. Our study presented some limitations. First, the cross-sectional study design means it is not possible to establish cause and effect relationships. Moreover, we presented an investigation from only one center and did not evaluate inflammation with a specific inflammatory marker. Thus, any generalization of the data presented for other HF patient groups should be made with due caution.
Although a previous study 8 considered that the application of secondary Cachexia Consensus criteria means we lose high risk patients due to reducing cachexia prevalence, in this study we verified that using the consensus criteria allowed for a distinction to be made between patients that present complex alterations which are characteristic of cachexia and those that only present weight loss. Applying the secondary criteria means we do not lose at-risk patients and allows for attention to be concentrated on cachectic patients in an institution with adequate treatment measures. Thus, we believe that patients who have only lost weight cannot be attributed the same gravity which cachexia represents, although they do obviously require attention and nutritional interventions.
CONCLUSIONS
A high prevalence of cardiac cachexia was observed and the affected patients presented increased loss of muscle mass, adipose tissue, low handgrip strength, a substantial presence of fatigue and anorexia, and biochemical alterations (anemia, raised PCR, and hypoalbuminemia), confirming the clinical deterioration in these patients. These results reinforce the need for cachexia to be routinely investigated so that preventative measures can be adopted and specific therapeutic interventions are implemented in the treatment of this syndrome.
Applying a new definition to cachexia allowed us a global overview of the modifications that occur in cachectic patients, and made it possible to identify the main altering factors in cachexia and those which were associated with this condition.