INTRODUCTION
Adequate iodine consumption during pregnancy for the prevention of fetal neurological problems has been the subject of many studies. In a previous study, Ferreira et al. assessed the nutritional iodine status of pregnant women in the state of Sao Paulo using the same population as studied here. Conversely, the objective of the present study was to assess the levels of urinary iodine excretion, thyroid function, antioxidants and oxidative stress markers in pregnant women. Thyroid stimulating hormone (TSH), anti-thyroglobulin (anti-TG) and anti-thyroperoxidase antibody (anti-TPO) levels were reported in the present study in order to complement the data for the assessment of oxidative stress 1.
Adequate iodine intake is a priority during pregnancy due to its impact on the formation of the fetus during pregnancy and also during the postpartum period. According to an estimate by the World Health Organization (WHO), approximately 1.9 billion people are at risk of developing iodine deficiency disorders (IDD). This is a public health problem on a global scale, characterized by high proportions of population groups with low iodine consumption 2.
The recommendations of the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) and the WHO define a urinary iodine excretion of 100 µg/l as normal and propose a daily requirement of 150 µg iodine for non-pregnant adults and 200 to 290 µg for pregnant and lactating women, respectively 3,4.
Iodine is considered to be a fundamental micronutrient for the formation of thyroid hormones. Dietary iodide is oxidized and converted to elemental iodine by the action of the enzyme iodinase peroxidase, which produces the hormone precursors monoiodotyrosine and diiodotyrosine. The union of two diiodotyrosine molecules forms thyroxine (T4) and the union of a diiodotyrosine and monoiodotyrosine forms triiodothyronine (T3), fundamental hormones for the regulation and release of thyroid stimulating hormone (TSH) 5,6,7. A normal thyroid gland does not show any difficulty in responding to this process of hormone formation. However, this is not the case when thyroid disease compromises the functional capacity of the gland or when healthy women who reside in iodine-deficient areas become pregnant 8.
Iodine has been reported to act directly as an antioxidant or indirectly by inducing antioxidant enzymes such as superoxide dismutase (SOD). Iodine may link to the double bonds of some polyunsaturated fatty acids of cell membranes, rendering them less reactive to free radicals. In addition, iodine competes with free radicals for lipid and protein membranes and DNA in order to induce cell stabilization. This antioxidant role is performed by oxidized iodine species obtained from the diet or by local deiodination 9,10,11,12. Thus, an adequate iodine intake and, therefore, its adequate availability for the performance of its functions in the organism are of extreme importance for all individuals, especially for an appropriate development of the fetus and for a balanced antioxidant status during pregnancy 12. Thus, an imbalance in the antioxidant system can lead to oxidative stress, characterized by excessive production of reactive oxygen species (ROS). The increase in ROS production in pregnant women leads to cellular damage, endothelial dysfunction, alteration in the processes of trophoblastic differentiation, causing problems in the placenta and thus, gestational hypertension. Research with pregnant women with hypertension revealed an increase in ROS levels and low levels of antioxidants, suggesting that free radicals can cause vascular damage in pregnant women 13.
The balance between reducing agents and the antioxidant system is essential. The antioxidant system may be enzymatic or non-enzymatic. An antioxidant is defined as any substance that, when present at low concentration compared to the concentration of the oxidizable substrate, regenerates the substrate or significantly prevents its oxidation 14. Enzymatic antioxidants are SOD, catalase (CAT) and glutathione-
peroxidase (GSH-Px), and non-enzymatic antioxidants are ascorbic acid (vitamin C), α-tocopherol (vitamin E), GSH, carotenoids, flavonoids and phenolic compounds, among others 14,15. Thus, adequate quantities of antioxidants in the intracellular medium are quite important for better security against attacks by these reactive species, preventing the onset of diseases and avoiding their complications, especially during pregnancy 16,11.
The oxidative metabolism is increased during pregnancy because of the high oxygen demand of mother and fetus and also because of a metabolic and hormonal overload, requiring more antioxidant components, dietary or not and enzymatic or not 10,16,17. Pregnant women with deficient or inadequate iodine intake undergo an even greater increase in the formation of free radicals due to the gestational process itself and to the reduced action of this micronutrient 12.
Thus, the objective of the present study was to determine the levels of urinary iodine excretion, the thyroid function markers and the biomarkers of oxidative stress in pregnant women.
PATIENTS AND METHODS
STUDY GROUPS
A descriptive cross-sectional study was conducted at the Department of Gynecology and Obstetrics of the Medical School of Ribeirão Preto - USP (FMRP-USP).
A group of volunteer pregnant women (n = 191) and a control group of non-pregnant women (n = 63) matched for mean age with the pregnant women were studied. Inclusion criteria were age over 18 years and time of gestation of up to 14 weeks (first trimester) for the pregnant women, and age over 18 years and being in the reproductive age range of 18 to 35 years for the non-pregnant women. Patients with a previous diagnosis of hypothyroidism, with chronic kidney disease, taking vitamin or mineral supplements or medications containing iodine in their composition, and patients with chronic diseases (diabetes mellitus, arterial hypertension, heart failure, and pre-eclampsia) were excluded from the study.
The sample was calculated considering the ioduria variable. Data from studies of pregnant women with and without iodine deficiency were used 12. For the variance, the largest variance found between groups was taken. Adopting a significance level of 0.05 and 0.80 of test power, the following equation was used to calculate the sample size:
where µ1 and µ2 are the means of each group.
The study was approved by the Research Ethics Committee of the institution (protocol 7477 of 10/11/2008) and all subjects gave written informed consent based on Resolution 196/96 of the National Health Council (CNS) 19.
ASSESSMENT OF NUTRITIONAL STATUS
Before performing anthropometry in the group of pregnant women, gestational age based on the date of last menstruation (DLM) was confirmed by consulting the prenatal care card and/or ultrasound data. The nutritional status of the pregnant women was classified according to the values proposed by the Institute of Medicine (IOM) and the nutritional status of the control women was determined using the values proposed by the WHO as a reference 20. BMI was calculated by the formula weight/height2 using the weight at the beginning of gestation.
DETERMINATION OF URINARY IODINE CONCENTRATION
Urinary iodine concentration was determined by mass spectrometry with inductively coupled plasma using an instrument equipped with a reaction cell (DRC-ICP-M ELAN® DR II, Perkin Elmer Sciex, Norwalk, CT, USA) operating with high purity argon (99.999%, Praxaair, Brazil). Each sample was placed in a 15 ml polypropylene Falcon® tube to a final volume of 10 ml (25 times dilution) with a solution of 1% TMAH containing 10 µg/l tellurium as the internal standard. The analytical calibration standards were prepared in the same diluent at a concentration of 0 to 100 µg/l. The curve was constructed by adjusting the matrix that contained the calibration standards.
The samples were then directly injected into the instrument and the results are expressed as µg/l. The quality control for the analyses was insured by analysis of the reference material 2670a Low. The analyses were carried out in the Laboratory of Toxicology and Metal Essentiality of the Faculty of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo.
The standard proposed by the WHO 3 for non-pregnant women was considered as a reference for the classification of urinary iodine concentration: < 100 µg/ml, insufficient; 100-199 µg/ml, adequate; and > 200 µg/ml, excess. The values considered for the pregnant women were: < 150 µg/ml, insufficient; 150-249 µ/ml, adequate, and > 250 µg/ml, excess.
DETERMINATION OF THYROID FUNCTION
Plasma TSH, free thyroxine fraction (FT4), total thyroxine fraction (TT4), anti-TG, and anti-TPO were determined by chemoluminescence (Immulite® 2000, DPC Cirrus Inc. Los Angeles, CA) in a single assay. The intra-assay errors calculated for TSH, FT4 and TT4 were 3.1%, 2.6% and 2.1% for TSH, FT4 and TT4 concentrations of 1.5 mIU/l, 1 ng/dl and 9.5 μg/dl, respectively. The inter-assay errors were 5.6%, 3.3% and 4.8% for TSH values of 0.2, 3.8 and 16.8 mIU/l; 11.4%, 3.9% and 3.9% for TT4 values of 12.7, 8.9 and 13.7 μg/dl; and 4.3% and 2.6% for FT4 values of 1.0 and 2.5 ng/dl, respectively 1.
MALONDIALDEHYDE
Malondialdehyde (MDA) was analyzed by the method proposed by Gerard-Monnier et al. 21. Serum MDA was determined using a 200 μl sample to which 650 μl of a 10 mM solution of 1-methyl-phenylindole in acetonitrile and methanol (2:1, v/v) and 150 μl of pure HCl (37%) was added. The Eppendorf® tubes were then shaken on a vortex mixer and incubated in a water bath at45 °C for 40 minutes. Next, the samples were cooled on ice and the tubes were centrifuged at 4,000 rpm for ten minutes. Absorbance at 586 nm wavelength was then read in the supernatant. MDA concentration was calculated by comparison to a curve of hydrolyzed 1,1,3,3- tetramethoxypropane (TPM). The analysis was performed in duplicate.
ADVANCED OXIDATION PROTEIN PRODUCTS (AOPP)
AOPP concentrations were determined in serum by the method of Witko-Sarsat et al. 22. A 40 µl amount of plasma diluted in 160 µl PBS was used and 20 µl acetic acid was then added to each well of a 96-well microliter plate (Becton Dickinson Labware, Lincoln Park, NJ, USA), followed by 20 µl of acetic acid. Ten microliters of 1.16 M potassium iodide (Kb, Sigma) were then added, followed by 20 µl of acetic acid. The absorbance of the reaction mixture was immediately read at 340 nm in a microplate reader against a blank containing 200 µl of PBS, 10 µl of KI and 20 µl of acetic acid. AOPP concentrations were calculated using a standard chloramine-T curve and expressed as µmol/l. The analysis was performed in duplicate.
REDUCED GLUTATHIONE (GSH)
GSH concentration was determined in serum by the method of Costa et al. 23. Samples of 25 µl were used, to which 1 ml Tris-EDTA (0.25 mol/l Tris base, 0.20 mol/l EDTA, pH 8.2) were added. For the preparation of one liter, 30,285 g Tris and 74,448 g EDTA were used. A first reading (A1) was taken and 25 µl DTNB (10 mmol/l in absolute methanol) were added. The compound was then shaken for 15 minutes and a second reading (A2) was taken at room temperature. The concentration of sulfhydryl groups was calculated using a standard GSH curve. The analysis was performed in duplicate.
FERRIC REDUCING ANTIOXIDANT POWER (FRAP)
FRAP was determined in serum using the assay developed by Benzi and Strain 24. Ten µl serum and 300 µl FRAP solution (0.3 M acetate buffer, pH 3.6, 10 mM TPTZ solution in 40 mM HCl and 20 mM FeCl3 solution) were pipetted on a 96-well microplate that was incubated at 37 °C for four minutes in a spectrophotometer, followed by reading at 593 nm. Sample concentration was based on a ferrous sulfate curve.
Reagents used for the determinations were: 300 mmol/liter acetate buffer, pH 3.6 (Riedel-de Haen(tm), Germany), and 16 ml C2H4O2 (BDH Laboratory Supplies, England) per liter of buffer solution, 10 mmol/liter TPTZ (Fluka(tm) Chemicals, Switzerland) in 40 mml/liter HCl, and 20 mmol/liter FeCl3-6H2O (BDH). The reagent was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ solution, and 2.5 ml FeCl3-6H2O solution. The analysis was performed in duplicate.
TOTAL ANTIOXIDANT CAPACITY (TAC)
Serum TAC was determined by the method of Erel 25 based on 2, 2-azinobis 3-ethylbenzothiazoline-6-sulfonate (ABTS). In this assay, ABTS is incubated with potassium persulfate to produce ABTS oxidation. Briefly, a 10 mg amount of ABTS was dissolved in 10 ml of an aqueous solution containing 2.5 mmol/l potassium persulfate and the mixture was left to stand in the dark at room temperature for one to four hours before use. For sample analysis, the oxidized ABTS stock solution was diluted with deionized water to an absorbance of 0.70 at 734 nm. After the addition of 1 ml of diluted oxidized ABTS to 10 µl of serum, the absorbance reading was taken ten minutes after the initial mixing. A 200 µl amount of reagent 1 (0.4 mol/l acetate buffer, pH 5.8) was added to 5 µl of serum, and the first reading (A1) was taken at 660 nm. Next, 20 µl of reagent 2 (ABTS in 30 mM acetate buffer, pH 3.6) were added and a second reading (A2) was taken five minutes later. The absorbance readings were compared to a standard Trolox curve, and the results are expressed as mmol Trolox equivalent/l. The analysis was performed in duplicate.
Superoxide dismutase (SOD)
SOD was determined in serum by the indirect nitroblue tetrazolium (NBT) method using the 19160 SOD® kit (Sigma-Aldrich Chemie GmbH®).
Preparation of working solutions: the WST working solution was diluted in 1 ml of WST solution with 19 ml of buffer solution. For the enzyme working solution the enzyme solution tube was centrifuged for five seconds and mixed by pipetting, and 15 µl of enzyme solution was diluted with 2.5 ml of dilution buffer. The SOD solution was diluted with dilution buffer to prepare the standard SOD solution as follows: 200 U/ml, 100 U/ml, 50 U/ml, 20 U/ml, 10 U/ml, 5 U/ml, 1 U/ml, 0.1 U/ml, 0.05 U/ml, 0.01 U/ml, and 0.001 U/ml. A 20 µl sample, 20 µl twice-distilled water, and 200 µl working solution were added to the plate, and 20 µl dilution buffer were added to each sample and to the blank. Next, 20 µl enzyme working solution were added to each sample and the blank and thoroughly mixed, and the plate was incubated at 37 °C for 20 min. Absorbance was read at 450 nm with a microplate reader. SOD activity (% inhibition rate) was calculated using the following equation: SOD activity (% inhibition rate) = {[(Ablank 1 - Ablank 3) - Asample - Ablank 2)]/(Ablank 1 - Ablank 3)} x 100. The analysis was performed in duplicate.
ANALYSIS OF VITAMIN E
Vitamin E was analyzed in serum using a method adapted from Araud 26, developed and standardized in the Bromatology Laboratory, Course of Nutrition and Metabolism, FMRP-USP.
Blood vitamin E profile was determined by HPLC. The sample was prepared by pipetting 200 µl serum in a test tube; 400 µl ethanol were added with thorough shaking, 400 µl hexane were added, the mixture was vortexed for 1 min, the tube was sealed with parafilm or an appropriate cap and it was centrifuged at 3,000 rpm for ten minutes. A 200 µl aliquot was removed from the hexane phase (upper), added to another tube, dried under an N2(g) flow, suspended in 200 µl of the mobile phase, and a 20 µl aliquot was injected into the HPLC apparatus.
A Shimadzu chromatograph, model LC-20AT was used, with a C-18 column (4.6 x 250 mm - 5 µm) and a visible UV detector model SPD-20A. The mobile phase consisted of acetonitrile:dichloromethane: methanol (7:2:1), the flow rate was 1.0 ml/min, with α-tocopherol detection at 292 nm. The concentrations were determined using an external standard and the results are expressed as µmol/l serum/plasma. The analysis was performed in duplicate.
STATISTICAL ANALYSIS
Exploratory data analysis was first performed to obtain a global view of the variables using tables with descriptive measures. Minimum and maximum median values and the 95% confidence interval were calculated, the data distribution was analyzed, and statistical tests were performed to determine group differences.
The quantitative variables were compared between groups using the t-test, with the level of significance set at p < 0.05, and the categorical variables were analyzed by the Chi-square test, with the results being presented as percentages.
The hypothesis of normal distribution was determined by the Kolmogorov-Smirnov test to assess the relationship between TSH and the numeric variables of the study group. When the hypothesis was rejected, the nonparametric Mann-Whitney test was used, and when it was not rejected, the t-test was used. The Chi-square test was used for the categorical variables and the Chi-square exact test was used when more than 20% of the cells showed an expected value of less than 5.
The hypothesis of normal distribution was determined by the Kolmogorov-Smirnov test to assess the relationship between ioduria and the numeric variables of the experimental group. When the hypothesis was rejected, the nonparametric Kruskal-Wallis test was used, and when it was not rejected, analysis of variance (ANOVA) was used.
RESULTS
Anthropometric assessment showed that the mean BMI of the control group (21.99 kg/m2) and of the group of pregnant women (25.2 kg/m2) was within the normal limits established by the WHO for the control group and overweight for the pregnancy group. Mean gestational age at the time of evaluation was 9.7 ± 2.9 weeks, with a median of nine weeks.
Table I. Oxidative stress markers of the control (n = 62) and pregnant women (n = 191) groups
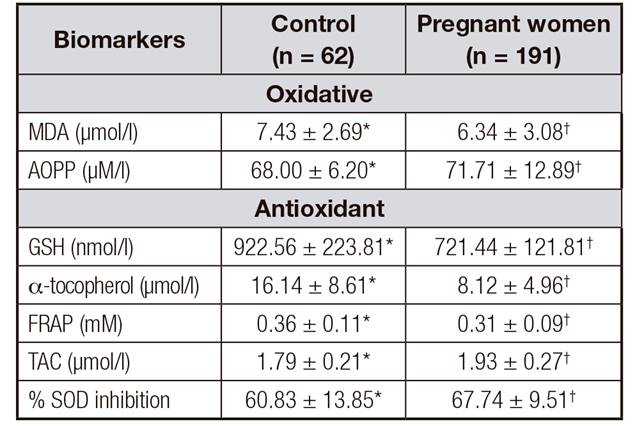
t-test. Values are expressed as mean ± standard deviation. Different symbols (*,†) p < 0.05. MDA: malondialdehyde; AOPP: advanced oxidation protein products; GSH: reduced glutathione; FRAP: ferric reducing antioxidative power; TAC: total antioxidant capacity; SOD: superoxide dismutase.
Hormone and ioduria levels did not differ significantly in the control and study groups in relation to TSH concentrations, which were unchanged in 89% of the women. Iodine insufficiency was higher in the pregnant women's group (48.5%), although it was also elevated in the control group (33.3%). Anti-TPO antibody concentrations differed significantly between controls (64.5%) and pregnant women (12.6%). Anti-TG levels were altered compared to reference values in 11.3% of the controls and in 3.7% of the pregnant women.
Table 2 presents the values of the oxidative stress markers. The MDA values of the controls were significantly higher than those of the pregnant women. AOPP differed significantly between groups, with higher values for the pregnant women compared to control. Regarding the antioxidant profile, GSH, α-tocopherol and FRAP values were significantly lower for the pregnant women (p < 0.05).
Table II. Oxidative stress markers in relation to the classification of normal and altered TSH values of the group of pregnant women (n = 191)
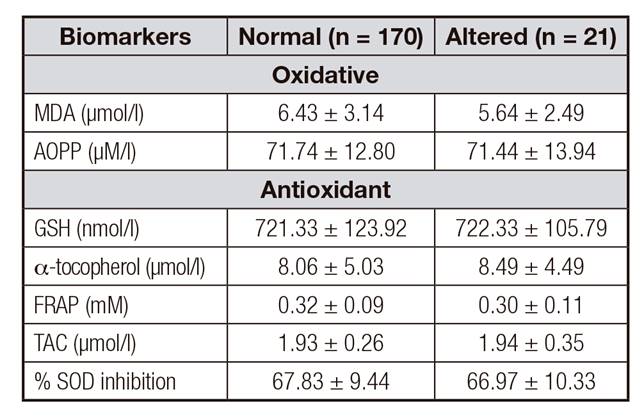
t-test. Values are expressed as mean ± standard deviation. Different letters (a, b, c) p < 0.05. MDA: malondialdehyde; AOPP: advanced oxidation protein products; FRAP: ferric reducing antioxidative power; GSH: reduced glutathione; TAC: total antioxidant capacity; SOD: superoxide dismutase.
The classification of TSH values for the pregnant women regarding the variables age, height and BMI did not show significant alterations. Also, no significant changes regarding TSH classification and its relationship with anti-TPO, anti-TG and ioduria levels were observed in either group (p > 0.05). Table 2 presents the relationship between oxidative stress markers and the classification of TSH values according to the reference index. No difference was observed in stress markers (MDA and AOPP) or antioxidants (GSH, α-tocopherol, FRAP, TAC and % SOD inhibition) among women with normal or altered TSH levels.
No significant changes in ioduria were observed in the group of pregnant women regarding variables such as age, weight, height, BMI and TSH. The classification of ioduria with respect to the TSH, anti-TPO and anti-TG values of pregnant women did not differ between the conditions of iodine insufficiency, adequate iodine levels or more than adequate iodine levels. Table 3 presents the data regarding the classification of ioduria and the oxidative stress markers. There was no difference in MDA or AOPP levels between the conditions of iodine insufficiency, adequate iodine levels or more than adequate iodine levels. Regarding the antioxidants, there was an increase in α-tocopherol levels among pregnant women which was more marked among those with adequate iodine levels than among those with more than adequate iodine levels. The pregnant women with iodine insufficiency showed lower α-tocopherol concentrations.
Table III. Classification of ioduria in relation to the variables of oxidative stress markers of the pregnant women group (n = 191)

Analysis of variance (ANOVA). Values are expressed as mean ± standard deviation. Different symbols (*, †, ‡) p < 0.05. MDA: malondialdehyde; AOPP: advanced oxidation protein products; FRAP: ferric reducing antioxidative power; GSH: reduced glutathione; TAC: total antioxidant capacity; SOD: superoxide dismutase.
DISCUSSION
A study assessing the nutritional iodine status of the same population of pregnant women was published before the present investigation, leading to the conclusion that pregnant women covered by the public health network of São Paulo had inadequate iodine concentrations classified as mild to moderate iodine deficiency 1. According to literature data, iodine deficiency may impair the formation of the fetus during pregnancy and the postpartum period 1,2. Based on the findings of the previous study and of literature data, analyses complementing the results of the nutritional iodine profile were conducted on the same population investigated by Ferreira et al. 1. Thus, the present study investigated the behavior of urinary iodine excretion and how an iodine insufficiency profile can affect thyroid function and oxidative stress biomarkers in pregnant women up to the 14th week of gestation.
Assessment of anthropometric parameters is an important tool contributing to the classification of the nutritional status of pregnant women. The BMI of controls was within the normal limits and overweight for the pregnancy group. An adequate BMI in association with a balanced diet regarding macro- and micronutrients helps prevent the onset of complications during the gestational period.
Iodine determination revealed that 81 of 191 pregnant women had iodine insufficiency. According to literature data, inadequate iodine levels may impair the production of TSH, causing the development of goiter 2. Also, an adequate nutritional intake of iodine is of primordial importance for the fetus during and after the gestational period 12.
However, the iodine deficiency detected in the pregnant women did not affect TSH or anti-TPO and anti-TG antibody levels. Studies have revealed that joint quantitation of anti-TPO and anti-TG antibodies can be used as a method for the diagnosis of autoimmune thyroid diseases. Women with high levels of these antibodies may be at higher risk of developing autoimmune thyroiditis during the puerperium 27,28,29. In a study of 127 pregnant women, Felipe et al. 30 observed that 11.3% of them had negative anti-TPO antibody levels. Thus, the lack of alteration of this parameter suggests that these pregnant women do not require treatment during gestation. Regarding TSH, its levels can become abnormal when thyroid hormones remain within values indicating a diagnosis of subclinical hypothyroidism and subclinical thyrotoxicosis 31.
The results of the classification of TSH values concerning anti-TPO and anti-TG antibodies did not reveal any difference between pregnant women with iodine insufficiency and those with adequate iodine levels. These data indicate that the level of iodine insufficiency of these patients did not induce changes in the antibody profile. The same can be suggested for ioduria levels since there was no significant difference in the case in question. Rebagliato et al. 32 assessed the levels of iodine intake in women and concluded that there was no association between iodine levels and TSH concentrations. The same was suggested by another study conducted by Soldin et al. 33.
Assessment of the oxidative stress profile of the control and pregnant women's groups revealed that the values of the oxidant marker MDA were higher in the control group than in the group of pregnant women. The opposite was observed for AOPP, whose value was higher among the pregnant women compared to control. Studies have indicated that oxidative metabolism increases during pregnancy due to the increased oxygen demand of mother and fetus, thus inducing free radical production 34.
Iodine deficiency is associated with pregnancy complications and it is a risk factor for pre-eclampsia and oxidative stress 12. Studies have pointed out that optimal levels of iodine intake induced an improvement of the antioxidant profile 9. In a study by Wu et al. 9, the group of pregnant women showed lower GSH, α-tocopherol, and FRAP levels compared to control. Conversely, TAC and SOD levels were higher in the group of pregnant women compared to control. In the present study, the iodine insufficiency observed in 81 pregnant women may have provoked a worsening of the antioxidant profile together with an increase of AOPP for this group. In addition, studies have indicated that iodination of arachidonic acid leads to the formation of iodine-lipids that act as ligands of the peroxisome proliferator-activated receptor gamma, regulating the expression of the SOD gene 35,36. The elevation of TAC and SOD in the present group of pregnant women suggests that adequate iodine levels are important for the equilibrium of oxidative stress during pregnancy.
Regarding TSH classification, the oxidative stress markers did not differ between pregnant women with iodine insufficiency and with adequate iodine levels. Regarding ioduria, an increase was observed in the activity of α-tocopherol, an antioxidant with high biological activity among women, with adequate iodine levels. Studies have shown that adequate iodine levels can reduce the oxidative stress status in pregnant women and that iodine may have an indirect action on enzymatic regulation 12,37. These data indicate that a diet with adequate iodine content can be a preventive factor of gestational complications.
On the basis of the results obtained, we conclude that mild to moderate iodine insufficiency did not induce changes in TSH or antibody levels. In addition, pregnant women with adequate urinary iodine excretion showed a better profile of the antioxidant α-tocopherol, indicating that iodine may play a significant role in antioxidant capacity during pregnancy and that its insufficiency may be harmful to the health of mother and fetus.















