INTRODUCTION
According to the World Health Organization (WHO), cancer is one of the main causes of death in the world. Lung, liver, gastric, colorectal, breast and esophagus cancer have the highest mortality and incidence rates. In 2012, it was reported that around 8.2 million people died as a result of this disease 1.
Around 40 to 80% of cancer patients develop malnutrition during their treatment; this could increase up to 90% in advanced disease 2. Malnutrition in cancer patients may occur due to different reasons: a) basic mechanisms of malnutrition; b) cancer cachexia; c) metabolic and digestive disorders; d) physiological disorders of the patient (anorexia-cachexia syndrome); and e) side effects of cancer treatment. Specially, gastrointestinal cancer may induce mechanical and functional complications, which could alter eating patterns in patients. In these types of cancer, the patients usually present a deteriorated digestion and malabsorption 3, leading to malnutrition.
Since it was established that cancer patients present a deteriorated nutritional status, a nutritional intervention has an important role in the treatment. The aim of nutritional support is to guarantee nutritional requirements, reduce micronutrient deficiencies, maintain muscle mass, and improve food intake and quality of life. In surgical patients, nutritional support therapy has an important role in the prevention and treatment of malnutrition and catabolism. Total parenteral nutrition (TPN) is indicated in the postoperative in the following circumstances: a) undernourished patients; b) patients poorly tolerating enteral nutrition; c) postoperative complications impairing gastrointestinal function in patients who are unable to receive and absorb adequate amounts of oral or enteral feeding for at least seven days; and d) gastrointestinal surgery. This support could be improved when it is supplemented with immunomodulatory nutrients such as glutamine. This amino acid may be considered when the patients require parenteral nutrition 4,5,6,7.
Glutamine (Gln) is the most abundant free non-essential amino acid in the body. Its primary source is skeletal muscle, but during catabolic status, infection, surgery or trauma, it is considered as semi-essential due to a depletion of its concentrations 8,9. The main functions of this amino acid are: a) main substrate for enterocytes; b) precursor of glutathione, the main endogenous antioxidant; c) prevention of bacterial translocation; and d) reduction of morbidity in surgery, sepsis, infection and trauma 10,11. Gln deprivation is related to muscle loss and reduced protein synthesis; therefore, Gln is conditionally indispensable in cancer, because it is characterized as being a hypermetabolic and hypercatabolic situation 13,14.
The aim of the study was to analyze the effect of parenteral glutamine in patients with gastrointestinal cancer who were undergoing surgery on nutritional status and gastrointestinal function.
PATIENTS AND METHODS
STUDY DESIGN
A prospective, interventional and longitudinal study was conducted between January 2015 and November 2017 at the Oncology Hospital of the (Instituto de Seguridad Social del Estado de Mexico y Municipios (ISSEMyM) in Mexico. Patients who entered the study met the following inclusion criteria: men and women over 18 years of age with primary cancer diagnosis (esophagus, gastric, colon, rectum, pancreas or liver), subjected to surgery, hospitalized and with indication of TPN minimum of seven days. Patients with renal failure, hepatic failure or with cachexia were not included in the study. Written informed consent was obtained from all patients and the study was approved by the local Committee of the hospital.
Patients were allocated in two groups by simple randomization method 15. Group 1 received only TPN (non-supplemented) and group 2 received TPN enriched with Gln dipeptide. The dose of intravenous glutamine supplementation was 0.4 g/kg/day and it was administered in the form of N(2)-L-alanyl-L-glutamine. The Department of Nutrition of the hospital did the calculation of the nutritional requirements for intravenous nutrition. The energy requirement was calculated by the Harris-Benedict formula and the protein intake was 1.5-2.0 g/kg of patient weight 16.
The measurements were performed at two different times: day one and day seven with the nutritional intervention with TPN after the surgery. The nutritional status was evaluated with the Subjective Global Assessment 16,17, which considers anthropometric, biochemical, clinical and dietetic data. In addition, blood samples were taken to analyze protein metabolism and a questionnaire was applied to measure gastrointestinal function.
ANTHROPOMETRIC MEASUREMENTS
Weight and body composition were measured with an electric bioimpedance bascule (Tanita(r), Bc533). The weight, muscle and bone mass was recorded in kilograms, while fat mass and water were expressed in percentage. Height was measured with a mobile Seca(r) stadiometer (Seca 213) and recorded in meters. Arm muscle circumference was taken with a metallic anthropometric tape and recorded in centimeters. The skin fold thickness was measured with Slim-guide(r) in millimeters.
BLOOD SAMPLE
Samples were obtained by venipuncture; glucose (mg/dl), urea (mg/dl), and creatinine (mg/dl) values were obtained by an enzymatic method. Prealbumin (mg/dl) was studied by immunonephelometry and albumin (g/dl) concentration was obtained by colorimetry. Lymphocytes (103/µl), monocytes (103/µl) and neutrophils (103/µl) were counted in an automated cell counter. All the analyses were performed using Roche(r) reactants.
QUESTIONNAIRE FOR GASTROINTESTINAL FUNCTION
The gastrointestinal function questionnaire includes four categories: a) stool frequency; b) stool consistency; c) stool emergency; and d) abdominal discomfort. Each category consisted of four items with a Likert scale where 4 points represent normal function; 5 to 8 points represent mild dysfunction; 9 to 12 points, moderate dysfunction; and 13 to 16 points, severe dysfunction (Appendix 1).
STATISTICAL ANALYSIS
The statistical analysis was performed using the SPSS version 22 (SPSS Inc., Chicago, Illinois). Baseline quantitative characteristics of the patients were represented by mean ± standard deviations. Categorical variables were displayed as frequencies and percentages. The Chi-square test was used to analyze the association between categorical variables and the t-test was used to compare groups between day 1 and day 7 after surgery.
RESULTS
Between January 2015 and September 2017, 70 subjects were recruited. Group 1 (non-supplemented with Gln) included 40 patients and group 2 (supplemented with Gln) included 30 patients. Table 1 shows baseline characteristics for the patients studied. There was no significant difference between both groups with regard to gender, oncologic diagnosis and anthropometric measurements. Before intervention, a physical examination was performed to measure clinical manifestations. The majority of patients presented micronutrient deficiency (70%), muscle wasting (62.5%) and dehydration (40%).
According to the anthropometric measurements (Table 1), the average tricipital skinfold was below the standard and the average arm circumference was within normal values in both groups. Body composition, mean visceral fat, bone mass, muscle mass and water percentage were normal in all patients. The weight loss percentage indicated that the majority of patients presented a severe risk of malnutrition in both groups.
Table I Patient baseline characteristics

BMI: body mass index. Comparisons were performed with the Chi-square test. *Statistically significant difference between groups, p < 0.05.
At the beginning of the treatment both groups presented more cases of severe and moderate malnutrition. After seven days with nutritional intervention, both groups improved their nutritional status (Table 2).
In group 1 (non-supplemented, day 7), the majority of the patients (44%) presented moderate malnutrition, but the cases with severe malnutrition decreased. In group 2 (supplemented with Gln), the majority of patients presented moderate (44%) and mild malnutrition (44%), and only 12% presented severe malnutrition. Therefore, the nutritional status was significantly better in the group supplemented with Gln (p = 0.008).
Table II Nutritional status after surgery

TPN: total parental nutrition. Comparisons were performed with the Chi-square test. *Statistically significant difference between groups, p < 0.05.
The treatment response of gastrointestinal function is shown in Table 3. At day one the majority of patients (44%) in group 1 presented mild dysfunction, and just one case presented normal function. In group 2, the majority of patients (48%) had moderate dysfunction compared to severe (36%) or mild (16%) dysfunction; no one presented normal function.
Table III Gastrointestinal function after surgery

TPN: total parental nutrition. Comparisons were performed with the Chi-square test. *Statistically significant difference between groups, p < 0.05.
After the intervention (day 7), severe dysfunction cases increased to 36% in group 1, while mild dysfunction cases decreased to 32%. In group 2 the majority of patients (80%) improved their gastrointestinal function. This improvement was observed in the cases of moderate and severe dysfunction that improved to mild dysfunction, reducing almost all cases of severe dysfunction. Therefore, there was a significant difference (p = 0.0001) between baseline and day seven.
Treatment response on protein metabolism is shown in Table 4. At the beginning of the treatment with TPN, both groups presented moderate hypoalbuminemia according to the average albumin value. At day seven there were no significant changes. According to the concentration of prealbumin, both groups increased its value, but only in the group supplemented with Gln the change was significantly different (p = 0.012). It is important to mention that in both groups prealbumin concentrations increased, but the average concentration was below normal clinical levels.
Table IV Protein metabolism after surgery
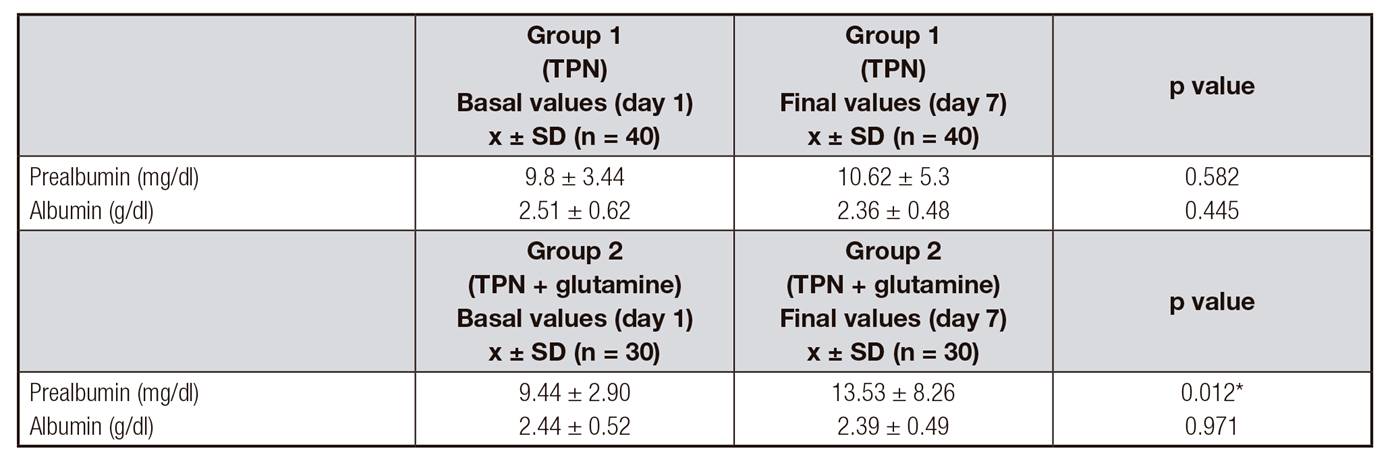
TPN: total parental nutrition. Results are expressed as mean ± standard deviation (x ± SD). Comparisons were performed with the t-test. *Statistically significant difference between groups, p < 0.05.
Table 5 shows the treatment response on blood cell markers. In the non-supplemented group, at day seven, no significant changes were found in the concentration of lymphocytes and monocytes. However, the neutrophil concentration decreased but there was no significant change. In the supplemented group with Gln, the average concentration of lymphocytes and monocytes increased at day seven, but only significant differences were found in the lymphocytes (p = 0.014). There was no significant change in the average value of neutrophils at day seven in this group.
Table V Nutritional cell markers after surgery
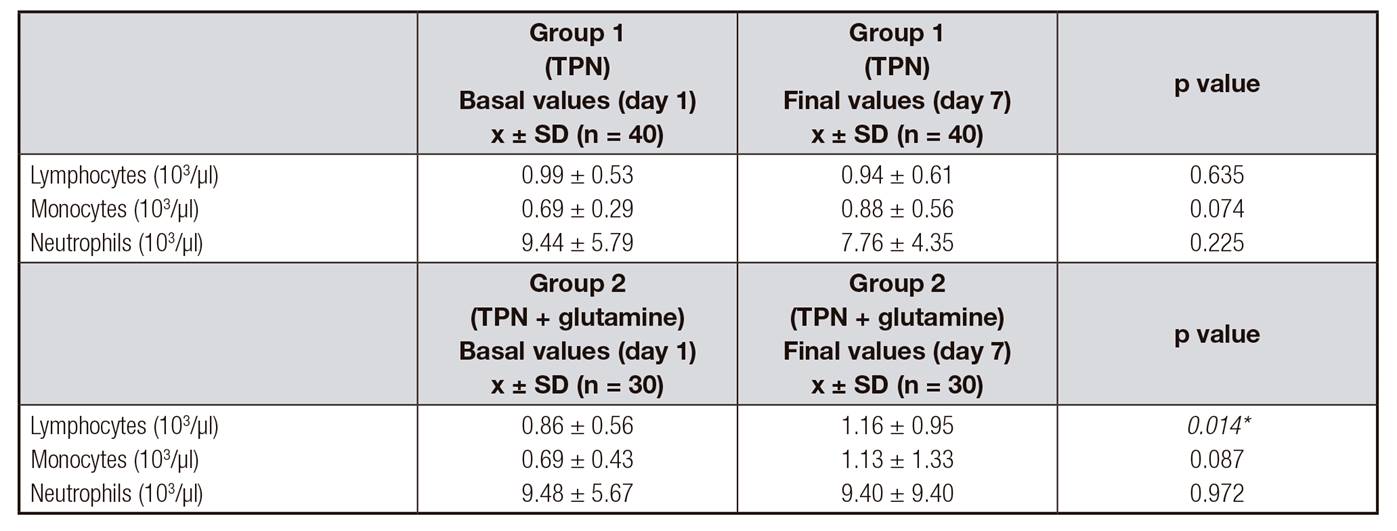
TPN: total parental nutrition. Results are expressed as mean ± standard deviation (x ± SD). Comparisons were performed with the t-test. *Statistically significant difference between groups, p < 0.05.
Both groups presented hyperglycemia at day one and seven. In addition, there were no significant changes in the average concentration of glucose at day seven. According to the average value of urea and BUN, both groups presented levels above normal on day one and day seven, without significant changes. Creatinine in the non-supplemented group significantly decreased (p = 0.019).
DISCUSSION
The results suggested that supplementation with Gln for seven days could improve nutritional status in patients with gastrointestinal neoplasia. It was found that the concentrations of prealbumin increased significantly in the supplemented group. However, the serum levels of albumin did not change in both groups. Since prealbumin is a marker of nutritional status with a half-life in plasma of two days, it is more sensitive to changes in protein-energy status than albumin 18. The group that received TPN enriched with Gln for seven days improved serum levels of prealbumin in postoperative gastrointestinal cancer patients as shown by Lu et al. 19.
Gastrointestinal tract provides a barrier against bacteria or toxins. However, cancer patients, as well as individuals who undergo surgery, have an increase in intestinal permeability related to bacterial translocation. This fact causes complications like sepsis, malnutrition or organ failure 20. It is known that Gln has an important role in the intestinal mucosa integrity because it is the main substrate for the intestinal mucosa cells. In cancer patients this integrity could be decreased 21,22. Our results showed that supplementation with Gln significantly improved gastrointestinal function of the majority of the patients. However, the non-supplemented group progressed to severe dysfunction after seven days. This could happen due to the fact that patients subjected to surgery have higher intestinal permeability, leading to bacterial translocation. Group 2 patients presented complications due to bacterial infection. A Gln-enriched enteral diet for seven days in critical ill patients could prevent gastrointestinal infections, but no significant differences were found between the supplemented and non-supplemented group. A study carried out by Conejero et al. showed similar results 23. It is worth mentioning that the gastrointestinal function study could be limited due to the distribution of diagnoses (type of cancer) and the number of patients per group.
Jun et al. demonstrated that parenteral glutamine supplementation in combination with enteral nutrition for seven days improves intestinal mucosa immunity. Also, it may contribute to the prevention and treatment of sepsis 24. Other studies support that Gln administration regulates the gut barrier function 23,24).
Cancer patients are usually immunosuppressed and present nutriment deficiencies. Additionally, they have more possibilities to develop septic complications than other patients. Gln is a precursor of purines and pyrimidines and also a primary substrate for lymphocytes 25. In our study, the concentrations of lymphocytes and monocytes improved significantly in the group supplemented with glutamine after seven days of intervention. However, neutrophil concentrations did not change. Chang et al. 26 have shown that Gln supplementation improves significantly lymphocyte proliferation stimulated by phytohemaglutinin.
Gln supplementation increased immune cells such as granulocytes and lymphocytes in malnourished abdominal surgery patients (preoperative and postoperative stage for five days). This was also shown by Asprer et al. 27. Besides, Manhart et al. demonstrated that glutamine supplementation in mice for ten days improves the gut immune system, preventing lymphocyte atrophy of Peyer's patches 28.
The main substrate for neutrophils is glucose. Vlessis et al. 29 suggested that neutrophils are able to use glutamine when the glucose is restricted. Despite the role of Gln in neutrophil is less defined, it is know that it increases the function of lymphocytes, macrophages and neutrophils exogenously during metabolic stress. However, more studies that could explain neutrophil behavior with glutamine are necessary. In fact, neutrophil concentration did not increase in this study.
TPN could usually cause metabolic alterations like hyperglycemia. Rosmarin et al. 30 demonstrated that TPN dextrose infusion rate was positively correlated with blood glucose concentrations. In fact, infusion rates over 4-5 mg/kg/min increase the risk of hyperglycemia. However, it is common that cancer patients are diagnosed with hyperglycemia due to a side effect of the treatment or an increase in use rate and liver production of glucose. In our study, only eight patients presented hyperglycemia, three of them had diabetes, three were related to a metabolic alteration due to TPN and two were subjected to Whipple surgery.
Gln is synthesized primarily in the muscle, being their main source 31. In hypermetabolic conditions like cancer, surgery, trauma or sepsis, the glutamine concentrations are depleted. This could explain why serum creatinine levels decreased in the control group due to muscle wasting, glutamine depletion and the absence of exogenous glutamine.
Cancer patients subjected to surgery and hospitalized are more likely to develop complications such as malnutrition. This circumstances could diminish quality of life and reduce treatment response. These data, in our opinion, support that glutamine supplementation in gastrointestinal cancer patients undergoing surgery is beneficial. These results substantially confirm the findings of other studies, which suggest that Gln have a positive effect on nutritional status, gastrointestinal function, protein metabolism and cell markers.
CONCLUSIONS
In this work, we found that glutamine could improve gastrointestinal function, diminishing diarrhea, urgency, abdominal pain and distention. When the gastrointestinal function gets better, nutrimental absorption increases, so nutritional status improves. In addition, glutamine has a positive effect in prealbumin, lymphocytes and monocytes concentrations which is reflected in a better nutritional status.
















