INTRODUCTION
In Europe, 27.9 % to 34.4 % of the patients admitted to general hospitals receive enteral nutrition (EN) therapy (1). A multicenter survey on the prevalence of hospital malnutrition found that only 5.6 % of patients receive EN in Latin America (2). A French study evaluating macronutrients delivered in an intensive care setting found that up to 78 % of its sample of 51 patients received EN (3). A 2008 Brazilian study of 907 elderly patients in intensive care found that 40 % received EN (4). In a study on the prevalence of drug-enteral nutrition interaction in a Brazilian intensive care unit (ICU), Reis et al. (5) found that 29 % of hospitalized patients received EN. A previous study by our group (6) found that the majority of ICU patients (59 %) did not receive EN. In fact, there have been no recent censuses on the subject, and consistent data about the number of critically ill patients who receive EN are unavailable, especially in Brazil.
Moreover, little is known about the conditions that facilitate or hinder the use of EN, i.e., that determine whether this therapy is fitting for some patients but not others. Some authors have tried to explain this. Patel et al. (7), in a phase-3 single-center pilot clinical trial, enrolled 31 patients who were on mechanical ventilation and had septic shock (using vasopressors), comparing patients on early EN vs. those who did not receive it. No differences were identified between the groups regarding baseline clinical characteristics except for age (years), which was higher in the early EN group (64 ± 14 vs. 56 ± 16; p = 0.02). The authors also found that the early EN group spent less time on mechanical ventilation [27 (24-28) vs. 14 (0-26) ventilator-free days; p = 0.009] and less time in the ICU (25 [14-27] vs. 12 [0-22] ICU-free days; p = 0.014).
Studies indicate that one limitation of EN in patients on vasopressors is the risk of intestinal ischemia. A recent review of nine studies reported large variability (from 0.3 % to 8.5 %) in the incidence of intestinal ischemia in this patient profile (8). Although the incidence of intestinal ischemia secondary to EN is low, it contributes to significant morbidity and high mortality rates, ranging from 46 % to 100 % (9).
Recent guidelines (10,11) indicate that hemodynamically stable critically ill patients who receive early EM survive longer. On the other hand, in patients on vasoactive drugs, mechanical ventilation or sedatives, there may be alterations in blood flow and peripheral vascular perfusion, affecting gastrointestinal motility and gastric emptying (12). Under such conditions, gastrointestinal disorders could additionally limit the use of EN (13).
Although some studies (14-16) show that up to 60 % of critically ill patients have gastrointestinal motility disorders (vomiting, diarrhea, increased gastric residue, and constipation), little is known about the role of EN in these disorders. Two studies have reported an association between EN and gastrointestinal disorders. In 37 Spanish ICUs, Montejo (16) evaluated the effect of a management protocol for preventing diet discontinuation and gastrointestinal disorders, including only patients on EN (n = 400). This author reported that gastrointestinal disorders were frequent (increased gastric residue [39 %], constipation [15.7 %], diarrhea [14.7 %], abdominal distention [13.2 %], vomiting [12.2 %] and regurgitation [5.5 %]), pointing out that patients with gastrointestinal complications received a lower volume of EN than those without gastrointestinal complications (63.1 ± 1.2 % vs. 93.3 ± 0.3 %; p < 0.001), had longer hospital stays (20.6 ± 1.2 vs. 15.2 ± 1.3 days; p < 0.01) and higher mortality (31 % vs. 16.1 %; p < 0.001). Nassar et al. (17) studied 106 surgical patients in a Brazilian ICU and found that constipation was common (69.9 %), as well as that early EN (within 24 hours of ICU admission) was a protective factor against constipation (OR: 0.16; 95 % CI: 0.05-0.45) (17). Other authors (15) have reported that gastrointestinal disorders are related to poor clinical outcomes. A multicenter study including patients (n = 377) from 40 ICUs in several European countries found that the number of concurrent gastrointestinal symptoms is an independent risk factor for 28-day ICU mortality (OR: 3.18; 95 % CI: 1.08-9.40; p = 0.035) (15). However, the causal relationship between EN and these gastrointestinal complications is still not clear, nor is their impact on hard outcomes.
Given the lack of robust evidence about the conditions that favor EN, as well as about how critically ill patients are affected by gastrointestinal disorders, the aim of this study was to describe the incidence of gastrointestinal disorders and to identify conditions associated with the use of EN in a cohort of critically ill patients.
METHODS
DESIGN, SETTING, AND POPULATION
At the end of 2016, a prospective cohort study was conducted at the ICU of a large public university hospital in southern Brazil to assess the incidence of and factors associated with constipation (6). Adults (aged ≥ 18 years) who remained in the ICU for a period ≥ 3 days were included. Adults who had constipation or diarrhea on admission, or who had preoperative bowel preparation with enemas, a colostomy, who were admitted from another ICU or were readmitted to the ICU during the current hospitalization were excluded.
DATA COLLECTION
At the start of the study, the first 10 ICU patients were included, who were followed up until ICU discharge or death, at which point new patients were admitted as participants. The patients were followed daily during the first 10 days of ICU stay by previously trained nurses who used a standardized instrument developed for the study, that consisted of variables related to previous and current clinical history, interventions and therapeutic support, nutritional support, and daily Acute Physiology and Chronic Health Evaluation (APACHE II) and Sepsis-related Organ Failure Assessment (SOFA) scores. For each gastrointestinal disorder, a criterion was adopted, as described below, according to the literature. Constipation was defined as no bowel movement for three consecutive days (17). Diarrhea was considered three or more episodes of liquid or semi-liquid stools per day (18). Vomiting was defined as the occurrence of any visible regurgitation of gastric content (19). Abdominal pain, distension, and the need for gastric decompression were determined through clinical assessment by the care team, and were recorded in the patients’ charts.
The study was conducted in accordance with the Declaration of Helsinki guidelines, and the hospital’s research ethics committee approved the research protocol.
STATISTICAL ANALYSIS
Data were tabulated and analyzed using the SPSS 20.0 software. A descriptive analysis was performed according to the variables’ characteristics and distribution, and the assumptions of the statistical tests. Continuous variables were expressed as mean ± SD or median [interquartile range] as indicated. Categorical variables were expressed as absolute and relative frequency. The analysis considered two groups: a) patients who received EN for at least 24 hours during their ICU stay, and b) patients who did not receive EN, i.e., who received their diet orally or parenterally, or who fasted during their ICU stay. Comparisons between groups were performed using the chi-square test with residual analysis adjusted for categorical variables, and Student’s t-test for continuous variables. Cox regression with log-rank test was used to estimate the hazard ratio (HR), and Kaplan-Meier analysis between the EN and non-EN groups and gastrointestinal disorders, adjusted for ICU stay. The gastrointestinal disorders included in this analysis were those that were significantly different in the univariate analysis.
To identify conditions associated with EN, a multivariate regression was performed with robust variance and binary outcomes to calculate the odds ratio, adjusted for confounders. The variables for the multivariate regression were selected from the univariate analysis, considering p < 0.20; p values < 0.05 were considered statistically significant.
RESULTS
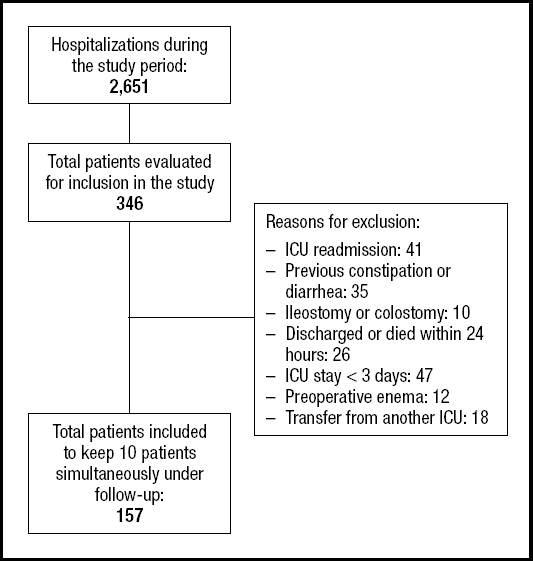
Of all ICU patients admitted during the study period (n = 2,651), 346 were potentially eligible. Of these, 157 were included, with 10 patients monitored at a time (Fig. 1).
The EN group had a more severe profile: they had a higher mean APACHE II score (23.6 ± 7.6 vs. 15 ± 7.2, p < 0.001) and a higher mean SOFA score on the day of ICU admission [7 (5-10.5) vs. 4 (2-6); p < 0.001], were admitted to the ICU for sepsis and neurological reasons (35.1 % vs. 10 % and 20.6 % vs 3.3 %, p < 0.001, respectively), and had higher ICU mortality (32 % vs. 10 %, p = 0.002).
Of all the included patients, 95 % had at least one gastrointestinal disorder during the study period. The most frequent disorders were constipation (75.9 %), abdominal distension (41.4 %), and diarrhea (28.7 %). Univariate analysis showed that any gastrointestinal disorder was more frequent in the EN group (97.9 % vs. 90 %, p = 0.02). In isolation, diarrhea and the need for gastric decompression were more frequent in the EN group (39.7 % vs. 11.7 %, p < 0.001 and 34 % vs. 13.3 %, p = 0.004, respectively). There were no significant differences between groups for the other variables (Table I).
Table I. Demographic and clinical characteristics

Values expressed as mean ± standard deviation, frequency (%) or median [25th percentile-75th percentile]. EN: enteral nutrition; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sepsis-related Organ Failure Assessment; ICU: intensive care unit; COPD: chronic obstructive pulmonary disease.
The risk of diarrhea (HR = 3.8, 95 % CI = 1.7-8.7) and the need for gastric decompression (HR = 2.8, 95 % CI = 1.3-6.0) was higher in the EN group. No difference in the risk of abdominal distension (HR = 1.6, 95 % CI = 0.9-2.7) was found between the groups, according to cumulative survival (Fig. 2).

Figure 2. Comparison of diarrhea (A), gastric decompression (B), and abdominal distension incidence (C) between patients who received enteral nutrition (solid line) and those who did not (dotted line).
The following conditions were independently associated with EN: neurological deficit (Glasgow Scale ≤ 9 or RASS Scale ≤ -2), prior enteral tube feeding, and SOFA score on the day of ICU admission (Table II). For each one-point increase in SOFA score, the use of EN increased by 20 %.
DISCUSSION
In a cohort of critically ill patients, the present study found that conditions associated with EN included neurological deficit, previous enteral feeding, and high SOFA scores. We also found that gastrointestinal motility disorders are extremely common in critically ill patients, with constipation and abdominal distension being most frequent. Moreover, the incidence of diarrhea and need for gastric decompression with a nasogastric tube was more frequent in the EN group than the non-EN group.
Although it is already known that the use of EN is more related to patients with neurological involvement, as they have a higher risk of dysphagia (20), no study was designed with the direct objective of evaluating the conditions associated with EN.
Since no study has directly identified the conditions associated with the use of EN in critically ill patients, it is impossible to draw any direct comparisons with our findings. However, indirect comparisons with studies designed for other purposes might be useful. For example, a prospective Spanish study (21) assessed the profile and costs of home EN, following patients for three years. Although the patient profile differed from ours, neurological deficits were also frequent (62 %) in their sample.
Likewise, the prolongation of and failure to resolve neurological conditions in critically ill patients requires that these patients become repeat users of EN (22). Thus, we verified that the previous use of enteral tube feeding was a condition frequently associated with EN on our cohort.
Patients with higher SOFA scores are more likely to not eat when alone (23), but no study showed a direct association with this clinical variable. Studies with objectives different from ours (21,23) indirectly describe a high SOFA score among patients with EN. To evaluate the effects of early EN on clinical outcomes, Khalid et al. (24) analyzed data from critically ill patients (n = 1,174) in a number of U.S. hospitals, divided into early or late EN groups. They found that the early EN group had higher APACHE II (23 ± 7 vs. 25 ± 8; p = 0.002) and SAPS II scores (52 ± 15 vs. 55 ± 16; p < 0.001). Although all patients in their study received EN at some point (early or late), there is some similarity between their results and ours regarding the association between SOFA score and EN use.
Critically ill patients undergo catabolic stress and systemic inflammatory response, which alter the morphology and function of the gastrointestinal tract (13), resulting in a higher incidence (> 60 %) of gastrointestinal disorders in ICU patients due to impaired motility, digestion, or absorption processes (14,15). An even higher rate of disorders was identified in the present cohort (95 %), which could be related to the fact that we included any type of gastric alteration (constipation, diarrhea, nausea, vomiting or abdominal distension).
Although, according to the literature, gastrointestinal disorders are frequent in critically ill patients, the reported incidence varies (16,17), including much lower rates than we identified. There are different explanations for this variability. The first of these refers to the set of signs and symptoms included in the studies, as well as lack of consensus about how to define these events. While Nassar et al. (17) define constipation as no bowel movement for three consecutive days, Nguyen et al. (25) described it using a strict concept that combined bowel movement frequency with clinical manifestations, which they called "impaired gastrointestinal transit". Variations in participant profile could also lead to different incidence rates. A multicenter study by Blaser et al. (15) evaluated patients on mechanical ventilation and, besides the disorders considered in our study, they considered gastrointestinal bleeding and abdominal hypertension. According to these authors, 60.2 % of their sample had at least one gastrointestinal disorder in the first week of ICU treatment. An older study reported higher rates of gastrointestinal disorders: in 1999, Montejo (16) evaluated 400 patients from 37 Spanish ICUs, finding that 62.8 % had gastrointestinal disorders. However, only critically ill patients on EN were assessed, and the included hospitals’ protocol was to avoid discontinuing EN and prevent the occurrence of gastrointestinal disorders.
There is also great variation in the literature when dealing exclusively with the incidence of constipation in critically ill patients (9 % [26] to 96 % [27]). In the present study the incidence of constipation was 75.9 % according to the previously used definition of no bowel movements for three consecutive days. Using the same definition, Nassar et al. (17) found a constipation incidence of 69.9 % in 106 surgical patients in a Brazilian ICU, which was similar to our finding in a different patient profile. In a retrospective U.S. cohort that included 83 patients with burns over more than 20 % of their body surface area, on mechanical ventilation in an ICU, late evacuation was defined as no evacuation after six days of ICU treatment, and the reported incidence was 36.1 % (28). Although there is large variability in the reported incidence rates, constipation is a common problem in critically ill patients.
In addition to frequent constipation, we found that abdominal distension affected 41 % of the patients in this study, with 10 days of follow-up. In a Chinese prospective cohort of 470 adults in 14 ICUs, with a median length of stay of 14 (11.0-14.3) days, abdominal distension occurred in 44.8 % of the patients (29). On the other hand, in a multicenter study, Blaser et al. (15) found a lower rate than ours (20.7 %), although their patients were followed for less time in the ICU (7 days).
Comparing gastrointestinal disorders between EN and non-EN patients, we found that diarrhea and the need for gastric decompression were more frequent in the EN group. In Montejo (16) and Heyland et al. (30), the main gastrointestinal complication found among critically ill patients on EN was increased gastric residue, which we also found. The incidence of diarrhea in the EN group was 39.7 %, which was significantly higher than in the non-EN group. A retrospective Australian cohort (n = 50) of critically ill patients on EN included stool volume in the definition of diarrhea, reporting an incidence of 78 %, which was higher than ours (31). However, their definition differed from ours, and the volume considered in their study was based on the subjective assessment of the nursing staff.
Despite being a finding already reported in other studies (32), monitoring the incidence of diarrhea during the infusion of EN allows care practices to be reviewed, since among the causes of this disorder are the composition of enteral formulas (high osmolarity or low amount of dietary fiber increase the risk), the characteristics of their administration, including the position of the enteral tube (gastric or jejunal, with no consensus on the benefit of a gastric tube in preventing diarrhea), and the mode of infusion (use of an infusion pump decreases the risk of diarrhea when compared to gravitational dripping) (33). Also, it is necessary to observe the risks of microbial contamination of the EN formulas used, as well as contamination of the enteral tube lumen, due to inadequate handling practices and lack of care with diet administration devices (34).
Although we found no difference in the incidence of constipation between the EN and non-EN groups, some authors have detected a difference. Nassar et al. (17) reported that early EN (within 24 hours of ICU admission) was associated with a lower incidence of constipation (OR: 0.16; 95 % CI: 0.05-0.45). In another study, late EN (OR: 3.42; 95 % CI: 1.88-6.22; p < 0.001), sedatives (OR: 3.07; 95 % CI: 1.71-5.52; p < 0.001) and surgery (OR: 1.86; 95 % CI: 1.01-3.42; p = 0.047) were independent risk factors for delayed bowel movement (35).
Our study was initially designed to assess the incidence of gastrointestinal disorders and their determinants in ICU patients, not to compare the relationship between these events and the risk of EN. Thus, other variables that could be predictors of EN may have been overlooked in our cohort. On the other hand, our study design was robust, with methodological care taken throughout its planning, implementation, data analysis and interpretation, and it provides information that is scarce in the literature.
CONCLUSION
Our data indicate that the conditions associated with EN in critically ill patients were neurological deficit, prior enteral tube feeding, and higher SOFA scores. Gastrointestinal disorders were very common, especially constipation and abdominal distension. Among the critically ill patients who received EN, there was a higher incidence of diarrhea and need for gastric decompression.