INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed type of cancer and the second cause of death from cancer globally (1). Nitroxidative stress (2), inflammation (3), and nutritional status (4) are suggested to be involved in its pathogenesis and progression.
Nitroxidative stress is characterized by an imbalance between antioxidant enzymatic (superoxide dismutase, catalase, glutathione peroxidase) and non-enzymatic (glutathione, carotenoids, tocopherols, vitamin C, flavonoids, among others) defense systems and the generation of reactive oxygen and nitrogen species (RONS) (5,6). RONS may even be classified in subforms according to intensity from physiological oxidative stress (eustress) to excessive/toxic oxidative burden (distress), and as acute, chronic, and repetitive oxidative stress (7). This redox imbalance can mediate the oxidation of biomolecules such as lipids, carbohydrates, proteins, DNA (deoxyribonucleic acid) (6), for instance in the generation of products like malondialdehyde (MDA) (2). Clinical studies unraveled that CRC patients have high levels of MDA and these are related to cancer stage (8).
It is well known that inflammation is related to nitroxidative stress (9) and CRC development/severity (3). Several studies have shown that the increased levels of some proinflammatory chemokines secreted by the tumor, such as interleukin (IL)-1, IL-6 and IL-8, are associated with carcinogenesis and can promote growth and migration of cancer cells (10,11). These cytokines are related to the presence of the tumor, its severity/cancer stage, and higher mortality (12). IL-6 (3), IL-8 (13), IL-17 (14), and TNF-α (tumor necrosis factor alpha) (15) are among these inflammatory mediators, and there are some shreds of evidence of their relationship with tumor stage and cancer prognosis.
Another relevant aspect in CRC progression is nutritional status and its crosslink with functional capacity (16). Tumor microenvironment and the activation of immune cells and systemic inflammation lead to catabolic signaling, which reduces appetite through the central nervous system, stimulates lipolysis and proteolysis that accelerates the loss of adipose and muscular tissue, and consequently impairs weight control and strength (11). Additionally, malnutrition is a predictor for severe complications and death in CRC surgical patients (4) and positively correlates with tumor stage (17).
As such, this study aimed to evaluate the association between nutritional parameters, oxidative and inflammatory biomarkers, and tumor progression in newly-diagnosed CRC patients treated at a public reference center in Maceió, Alagoas, Brazil.
METHODS
STUDY DESIGN
A cross-sectional study was conducted from July 2017 to January 2019 at the Professor Alberto Antunes University Hospital (HUPAA), located in Maceió, Alagoas, Brazil, a public reference center for cancer treatment. The Ethics Research Committee of the Federal University of Alagoas approved the project under number 1.796.339.
STUDY GROUP
In this study patients were included with the following criteria: 1) newly diagnosed with CRC by a histopathological exam; 2) age ≥ 18 years, both sexes; 3) undergoing clinical follow-up at HUPAA. Non-inclusion criteria were: 1) previous surgery, chemotherapy and/or radiotherapy; 2) severe general conditions; 3) renal or hepatic dysfunction; 4) pregnant and lactating women.
EQUIPMENT
We used the following: a Sanyo VIP Series biofreezer; high-performance liquid chromatography (HPLC) coupled to a UV detector (Shimadzu®, serial no. L201550); a spectrofluorometer by Thermo Scientific® (Multiskan); a Filizola® Welmy digital balance with a coupled stadiometer; a scientific adipometer and inextensible measuring band by Lange®; and a Jamar® hydraulic dynamometer.
BLOOD SAMPLES
The collected blood was stored in a tube containing EDTA and was centrifuged at 4,000 rpm for 10 minutes at 4 °C. The supernatant was removed and stored at -80 °C for later biochemical analyses.
OXIDATIVE STRESS BIOMARKERS
MDA peak was measured by HPLC (high-performance liquid chromatography) according to Vickie et al. (1990) (16). The reading time was 6 min, where MDA retention time is around 2 min 51 sec, and the UV detector was set at 270 nm. MDA was expressed as ng/µL.
INFLAMMATION BIOMARKERS
IL-6, IL-8, IL-17 and TNF-α were analyzed in duplicate by enzyme-linked immunosorbent assay (ELISA) following the manufacturer's instructions (PeproTech® kit, PeproTech Brasil FUNPEC, Ribeirão Preto, SP, Brazil) and results were expressed as pg/mL.
NUTRITIONAL ASSESSMENT
The weight and height of adults and the estimated height according to knee height of the elderly (17) were measured; then BMI (body mass index) was calculated, expressed in kg/m2, and the appropriate cutoffs were used (18,19). Usual weight was reported by the patients and considered as the weight at 6 months before diagnosis.
Arm circumference (AC) and triceps skinfold (TSF) were obtained according to Lohamn et al. (1991) (20), and arm muscle circumference (AMC) was calculated. AC, TSF and AMC were expressed as adequacy (%) of percentile for comparisons (20-22).
FUNCTIONAL ASSESSMENT
Handgrip strength (HGS) was collected according to Luna-Heredia et al. (2005) (24), with three consecutive measurements in the dominant and non-dominant hands. The data regarding force in the dominant hand were considered for comparisons and expressed as kg/force.
Anthropometric/functional measurements were not collected in the following cases: patients with a venous access at the place of measurement; patients with some limb amputation/immobilization; patients who had edema on the day of the consultation; and patients unable to perform the HGS.
CANCER STAGE EVALUATION
Patients were classified in stages according to the American Joint Committee on Cancer criteria (25), that is, in 0, I, II, III or IV stage, depending on the tumor-node-metastasis (TNM) staging, and were grouped as group 1 (stage 0-III) and group 2 (stage IV).
STATISTICAL ANALYSIS
The statistical analysis was performed using the SPSS® version 20 software. Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR), and categoric variables as frequency [n (%)]. The Mann-Whitney test was used for comparisons of median values. Next, a binary logistic regression analysis was performed between nutritional and biochemical biomarkers, one by one, and cancer stage as adjusted for sex and age. Data were expressed as 95 % confidence intervals (CI) and odds ratios (OR). Significance was considered when the p-value was < 0.05.
RESULTS
Twenty-eight newly-diagnosed CRC patients were included in this study, in which fourteen (50.0 %) were male and fourteen (50.0 %) were female, with a mean age of 59.0 ± 15.6 years. Twenty patients were included in group 1, of which two (7.1 %), four (14.3 %), nine (32.1 %), and five (17.9 %) were in stages 0, 1, 2 and 3, respectively, and eight patients (28.6 %) were in group 2 (TNM stage IV). Other general data are listed in table I.
Table I. General data of newly-diagnosed colorectal cancer patients at a University Hospital in Maceió, Alagoas, Brazil; collected from July 2017 to January 2019
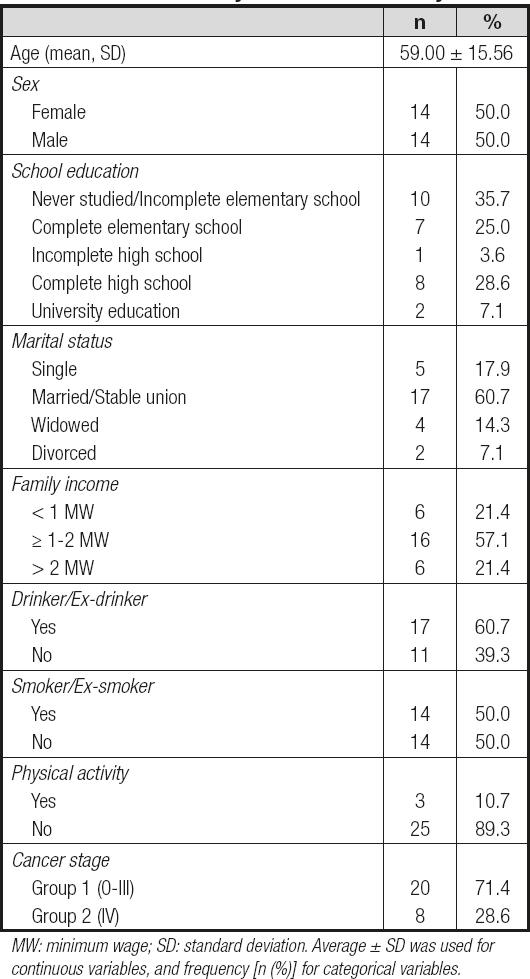
MW: minimum wage; SD: standard deviation. Average ± SD was used for continuous variables, and frequency [n (%)] for categorical variables.
According to table I, patients in group 2 had a significantly lower TSF adequacy [55.11 (21.23) vs 87.1 (38.55); p = 0.009] and higher serum IL-6 levels [5967.96 (1763.41) vs 2519.93 (1535.73); p = 0.002] when compared to group 1. No other nutritional measure or biochemical biomarker had a statistic association with tumor stage.
After the logistic regression adjusted for sex and age, TSF adequacy and serum IL-6 levels remained associated with the worst stage of CRC among newly-diagnosed patients. According to table III, while a reduction of 1 % in TSF adequacy enhanced by 0.09 % the chances of a patient being in stage IV (OR = 0.929; 95 % CI; 0.870 to 0.993; p = 0.029); every increase in serum IL-6 by 1 µg/mL enhanced by 0.1 % the chances of these patients being in stage IV (OR = 1.001; 95 % CI = 1.000 to 1.002; p = 0.012).
Table II. Nutritional assessment and biochemical serum biomarkers, according to tumor stage, of newly-diagnosed colorectal cancer patients treated at a University Hospital in Maceió, Alagoas, Brazil; collected during July 2017 to January 2019
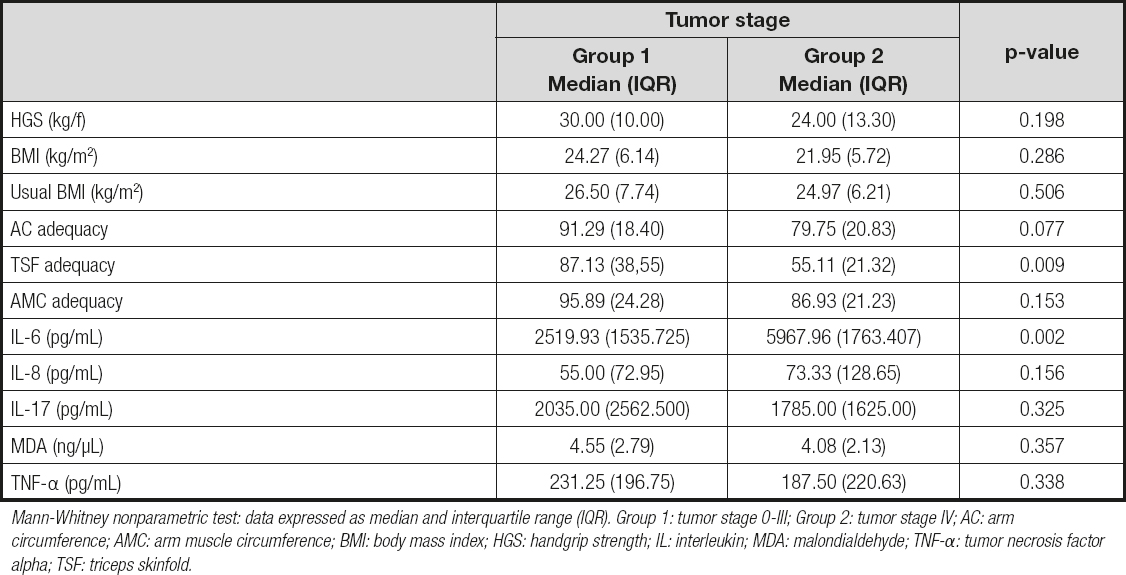
Mann-Whitney nonparametric test: data expressed as median and interquartile range (IQR). Group 1: tumor stage 0-III; Group 2: tumor stage IV; AC: arm circumference; AMC: arm muscle circumference; BMI: body mass index; HGS: handgrip strength; IL: interleukin; MDA: malondialdehyde; TNF-α: tumor necrosis factor alpha; TSF: triceps skinfold.
Table III. Association of anthropometric, functional, inflammatory, and oxidative variables with cancer stage of newly-diagnosed colorectal cancer patients at a university hospital in Maceió, Alagoas, Brazil, from July 2017 to January 2019
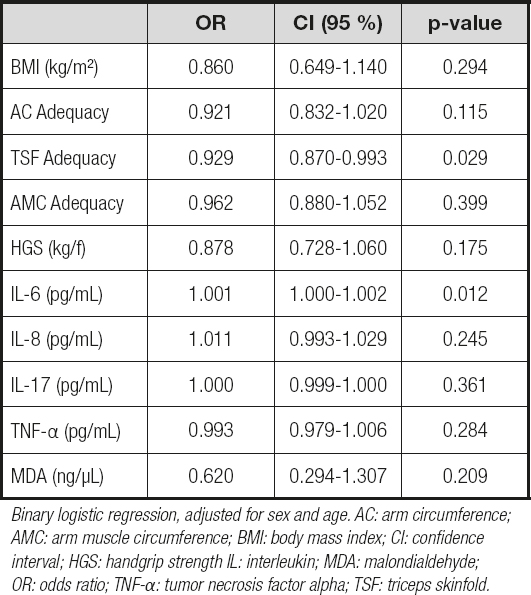
Binary logistic regression, adjusted for sex and age. AC: arm circumference; AMC: arm muscle circumference; BMI: body mass index; CI: confidence interval; HGS: handgrip strength IL: interleukin; MDA: malondialdehyde; OR: odds ratio; TNF-α: tumor necrosis factor alpha; TSF: triceps skinfold.
DISCUSSION
In this study, patients newly diagnosed with stage-IV CRC had the highest serum IL-6 levels and lower TSF adequacy values when compared to those with earlier stages (0-III). These results show that the depletion of adipose tissue proved to be more impacting than muscle catabolism or reduced strength for greater severity of tumor stage. Besides, IL-6 was the only cytokine that was associated with the worst prognosis in these patients, being in this respect more important than TNF-α, IL-8 and IL-17. Similarly, lipid peroxidation did not change with stage tumor progression.
The literature shows delayed diagnoses in CRC patients since it is a silent type of cancer, and most patients frequently underestimate early symptoms such as changes in bowel rhythm (23). A late diagnosis consequently implies cancer progression, disease activity, and hormonal alterations that involve fat mass depletion (24).
According to tumor staging, a relationship of lower adipose reserve - observed in this study as TSF adequacy - was observed by Agustsson et al. (2012). These authors compared changes in the body composition of newly-diagnosed oncology patients with intestinal obstruction or cachexia. They observed a significant reduction in adipose tissue in both groups, but they found no significant change in muscle mass (25). These findings reinforce the idea that adipose tissue depletion precedes loss of muscle tissue and strength (24,26).
In this study serum IL-6 levels have been associated with the worst tumor stage. Similar data were obtained by Zeng et al. (2017). According to them, IL-6 expression has a positive correlation with tumor stage, and this highlights the importance of these cytokine levels for cancer prognosis (3).
IL-6 is a cytokine produced by monocytes and macrophages (27), and is associated with inflammatory diseases such as cancer (3). Transcription factors mediate its inflammatory effects as NF-κb (nuclear factor kappa B) and STAT3 (signals through transducers and transcription 3) activator. Inflammation mediated by IL-6/NF-κb/STAT3 plays a necessary signaling role in tumor induction. Animal models suggest that IL-6 expression was increased in both the serum and tumoral tissue, and this expression occurred concomitantly with STAT3 activation (28). This tumoral induction promoted by IL-6/STAT3 was also confirmed by in vitro studies. Wang et al. (2019) analyzed human CRC cells and observed that IL-6/STAT3 signaling facilitated CRC cell proliferation (29). This fact may be explained by the ability of this cytokine to promote migration and angiogenesis, and to increase the occurrence of metastasis (10). In this way, in the sudy by Zeng et al. (2017) that analyzed 50 CRC tissue samples, and compared them to the adjacent mucosa, the authors found that IL-6 expression was associated with invasion depth and lymph node commitment, demonstrating the influence of IL-6 levels on CRC metastasis (3).
Furthermore, serum IL-6 levels have been associated with the worst tumor stage, and may be related to lower survival, as observed by Hara et al. (2017), who evaluated the levels of IL-6 in 113 patients with metastatic CRC before chemotherapy. These authors observed a significant reduction in overall survival and progression-free survival among patients with high serum IL-6 levels (30). A similar result was established by Xu et al. (2016), who reported a lower survival in those patients with higher levels of IL-6 (16.6 vs 26.0 months) (12).
Differently from those findings, in the results reported by Yamaguchi et al. (2019), who analyzed 27 different plasma cytokines, among the cytokines that were altered in CRC, as compared to controls, IL-8, IL-17A, and TNF-α were significantly enhanced in the plasma of CRC patients, but not so IL-6 (31).
Besides, the literature has shown that MDA could be higher in patients with advanced cancer, thus being a predictor of cancer (32). We did not find any association between oxidative damage as measured by this biomarker and cancer stage, a fact that may be explained by the recent diagnosis of the study patients. Similarly, Janion et al. (2020) did not show any differences in MDA levels according to cancer stage in CRC patients (33). In turn, it is important to highlight that the intensity of lipid peroxidation can be influenced by lifestyle (34), pre-existing chronic diseases, age, and tumor location (33).
Considering the complexity of CRC (different populations studied, different stages of disease), associated with the various inflammatory mechanisms involved in the onset and progression of the disease, a comparison of results between the various studies available represents a challenge.
CONCLUSION
The increase in IL-6 levels and the reduction in TSF adequacy enhanced the chances for newly-diagnosed CRC patients of having an advanced cancer stage, showing that inflammatory biomarkers and adipose tissue measurements can be useful for CRC prognosis, and may contribute to CRC screening and diagnosis. The oxidative damage biomarker (MDA), muscle mass (AMC), current weight (BMI), and functional assessment (HGS) were not found to correlate with cancer stage or severity.
Despite a small sample size, the present study suggests the power of IL-6 and TSF, and supports that inflammatory biomarkers and adipose tissue measurements can help healthcare professionals identify advanced stages of cancer in clinical practice, detecting deficiencies early and also contributing to nutritional cancer care.
Additionally, IL-6 could be a therapeutic target for cancer treatment. Further studies need to be conducted in patients newly diagnosed with CRC, and with advanced-stage CRC, to delimit a cut-off point for different populations, as well as the impact of inflammation modulation in cancer on disease progression and body composition.














