INTRODUCTION
Obesity has reached epidemic proportions representing a public health problem worldwide, with approximately 38 % of the adult world population estimated to be overweight by 2030, and severe obesity (when body mass index or BMI ≥ 40 kg/m2) is associated with chronic diseases and comorbidities (1). In obesity, elevated inflammatory markers such as C-reactive protein (CRP) (2), gamma-glutamyl transferase (GGT), and alanine aminotransferase (ALT) (3), are observed together with nonalcoholic fatty liver disease (NAFLD) (4). Additionally, adiposity reportedly affects the brain and reduces cognitive abilities (5), being associated with a higher risk of developing neurological disorders such as cerebral gray matter atrophy and Alzheimer's disease (6). Obese persons also score lower in cognitive testing and tasks of attention, memory, and executive function (7).
Bariatric surgery is an effective and increasingly popular method for weight loss among severely obese people. However, up to one third of patients either fail to lose weight or experience significant weight gains at follow-up (8). Nevertheless, bariatric surgery is associated with cognitive improvement - particularly memory and psychological health - 12 weeks and 24 months after surgery (9). However, cognitive impairment may also contribute to suboptimal weight loss after bariatric surgery. Patients with obesity frequently display deficient attention, executive functioning skills, and memory when compared to eutrophic individuals (10). Additionally, higher BMI is associated with worst performances on response inhibition, working memory, planning, and cognitive flexibility (10).
The long-term effects of bariatric surgery on cognitive function remain poorly understood. Additionally, until this moment we did not observe previous studies that have investigated the association between blood biomarker changes, neuropsychological deficit, and postoperative nutritional status. We hypothesized that, after bariatric surgery, patients with obesity would exhibit enhanced cognitive function, which could be dependent on biomarker improvements. Thus, the present study aimed to assess the neuropsychological function of patients with severe obesity 6 months after bariatric surgery. Moreover, we analyzed if neuropsychological function improvement was associated with changes in the patients' nutritional profiles.
MATERIALS AND METHODS
STUDY DESIGN AND PARTICIPANTS
This was a longitudinal prospective study. The sample comprised 22 patients with severe obesity (BMI ≥ 40 kg/m2) aged 18 years or older, recruited according to the number of patients treated at the Bariatric Surgery Clinic. The chosen sample included patients who had data available for all the variables investigated at preoperative baseline and 6 months after surgery. All participants underwent a Roux-en-Y gastric bypass (RYGB) (11), and all surgeries were performed by the same surgeons without postoperative complications. Inclusion criteria were BMI ≥ 40 kg/m2 and 18 years of age or older. Exclusion criteria were smoking, excessive alcohol consumption, pregnancy, malignant neoplasms, unresolved psychiatric illnesses, neurological damage, having no formal education, and impossibility to follow-up. All participants signed informed consent forms, and all procedures were approved by the Ethics Committee (process number 8763/2009) and started only after obtaining an informed consent.
ANTHROPOMETRICS AND BIOCHEMICAL MEASUREMENTS
Anthropometric data were analyzed at baseline and 6 months after surgery. The variables weight (kg), height (m), BMI (kg/m2), change in BMI (kg/m2), and total weight loss (%TWL; kg and %) were obtained through standardized protocols. Blood and urine samples were collected from all subjects, at baseline and at 6 months after surgery, for chemistry and hematology testing.
NEUROPSYCHOLOGICAL TESTS
Neuropsychological tests were applied to all volunteers at baseline and 6 months after surgery by the same investigator. The battery of tests demonstrated a high sensitivity to the altered cognitive performance of obese individuals (10) and bariatric surgery patients (12). The tests also assessed performance in multiple cognitive domains and were completed in approximately 60 minutes. The specific tests comprised the following we expose.
Semi-structured interview and mental state
The 30-item Mini-Mental State Examination (MMSE) screening test assessed temporal and spatial orientation, registration, memory, attention, calculation, language, recall, and constructive skills (13).
Beck's Anxiety Inventory (BAI)
Assessed the presence and severity of anxiety. The items described the emotional, physiological, and cognitive symptoms of anxiety, but not of depression (14).
Stroop Color and Word Test (SCWT)
This tool assessed mental flexibility, selective visual attention, and inhibitory control. The subjects performed color (SCWT 1) and color-word trials (SCWT 2); with the scores from the two trials, the number of correct responses to each set were obtained (SCWT 3) (15).
Concentrated Attention Test (TEACO-FF)
The scores were obtained by the result of the stimuli that the person should mark and actually scored, subtracted from errors (stimuli that should not be marked and were) and omissions, that is, the target stimuli that were not marked by the person (16).
Rey-Osterrieth Complex Figure (ROCF)
It is a qualitative test that assesses the skills of visuospatial organization, planning, and strategy development skills, as well as visuospatial, immediate (short-term), and late (long-term) memory. This analysis included copy accuracy and delayed recall accuracy (17).
Rey Auditory-Verbal Learning Test (RAVLT)
This instrument measured episodic declarative memory, immediate memory, new verbal learning, retention of information, and memory recognition (18).
Wechsler Adult Intelligence Scale-Third Revision (WAIS-III)
This scale assessed verbal understanding, perceptual organization, working memory, and processing speed. It provided the executive intelligence quotient (EIQ) and processing speed index (PSI) (19).
STATISTICAL ANALYSIS
All the data were described as median, minimum, maximum, or interquartile range. All neuropsychological, anthropometric, and biochemical analyses were performed through the Wilcoxon rank-sum non-parametric test for paired samples. Spearman's rank correlation coefficient test was used to assess the relationship between neuropsychological tests and biochemical data that reached statistically significant differences. For this analysis, the difference between post-test and baseline was obtained. The margin of error used in the statistical tests was 5 %.
RESULTS
Table I describes the demographic and medical characteristics of subjects.
Consistent with clinical interpretation, anxiety symptoms were absent during the assessment period (raw score of ~ 1 on BAI). The MMSE screening verified that no patients presented incapacitating neurocognitive disorders (median z-score = -0.15).
Table I. Baseline data in the screening of demographic and medical characteristics (n = 22)
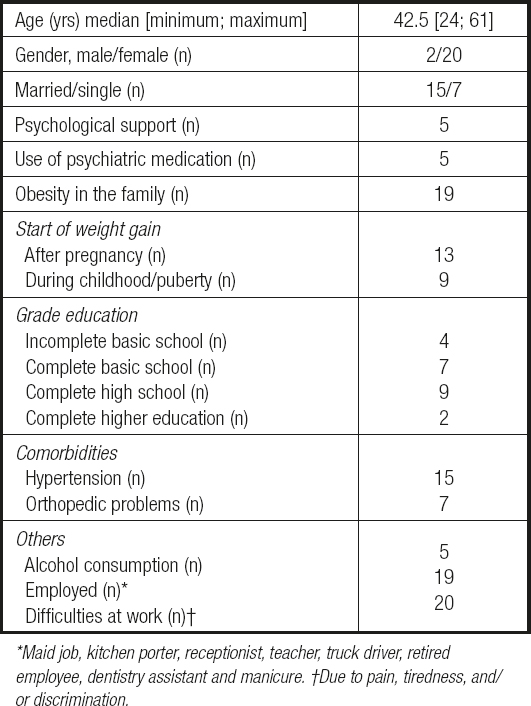
Maid job, kitchen porter, receptionist, teacher, truck driver, retired employee, dentistry assistant and manicure. †Due to pain, tiredness, and/ or discrimination.
Six months after the surgery, TWL and BMI decreased significantly (by approximately 22 % and 21 %, respectively). Additionally, the mean BMI values of the participants fell within moderate obesity (BMI = obese class I). Approximately, 31 % of participants exhibited a TWL greater than 25 %; only one participant presented a TWL of less than 10 % (Table II).
Table II. Anthropometric data of obese patients before RYGB and 6 months after RYGB (n = 22)
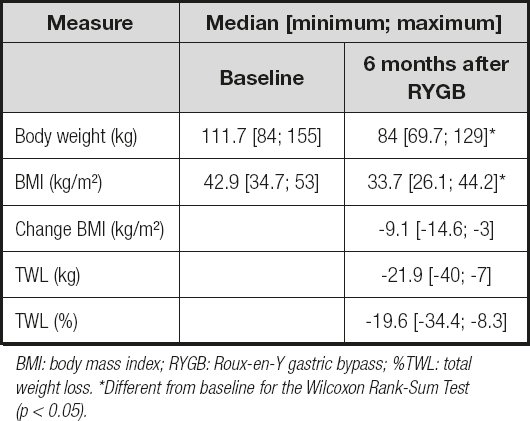
BMI: body mass index; RYGB: Roux-en-Y gastric bypass; %TWL: total weight loss. *Different from baseline for the Wilcoxon Rank-Sum Test (p < 0.05).
In most of the neuropsychological tests, the patients performed within the low-average range considered standard for their age, and no participant presented cognitive domain impairments. Attention, mental flexibility, and inhibitory control improved significantly (by approximately 12 %) on the SCWT 2. The other attention domains presented no statistical differences (Fig. 1).
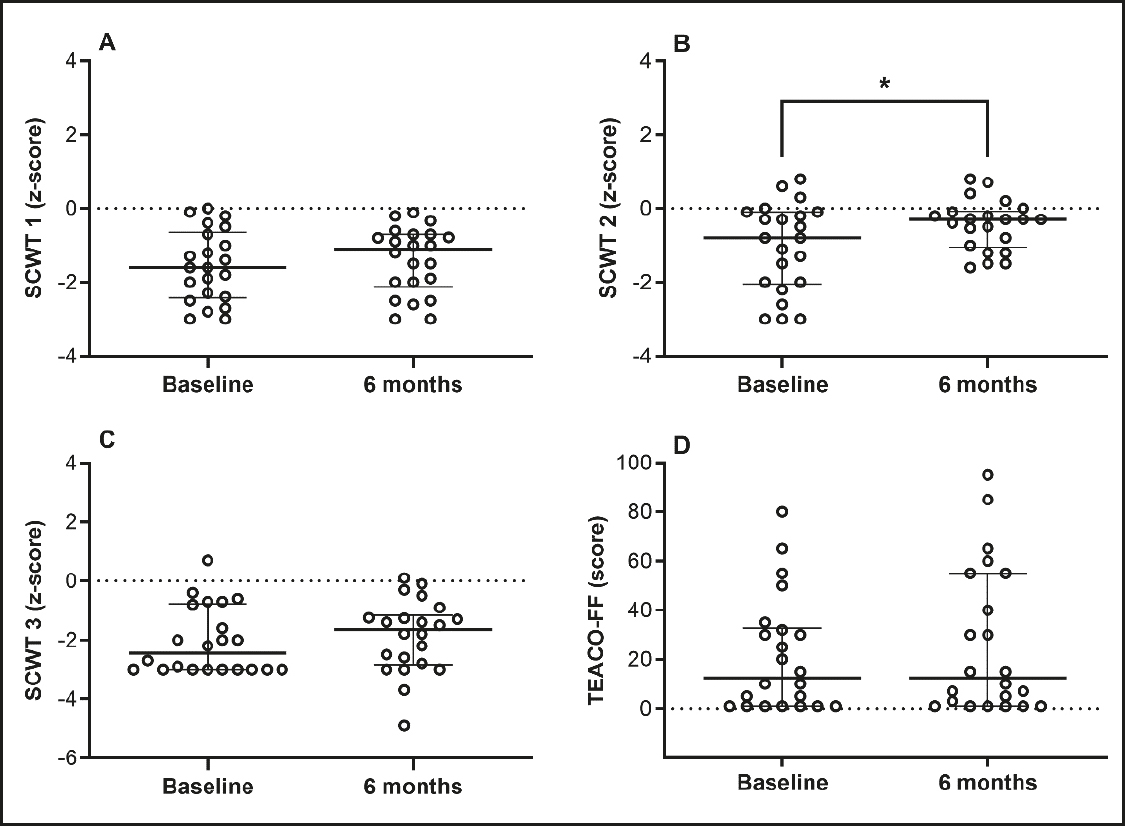
Figure 1. ffAttention, mental flexibility, and inhibitory control (SCWT: Stroop color and word test (panels A, B and C); TEACO-FF: concentrated attention test (panel D). *Different from baseline for the Wilcoxon Rank-Sum Test (p < 0.05). Data are median and interquartile range, n = 22.
Visuospatial organization, planning, visual, short- and long-term memory, and language presented no statistical differences at the 6-month postoperative assessment (Fig. 2A and 2B). Similar results were obtained for episodic memory, verbal learning, retention of information, memory recognition, and executive functions (Fig. 2C, 2D, and 2E). However, the PSI presented a lower variability and increased significantly by approximately 12 % at the 6-month postoperative cutoff (Fig. 2F).
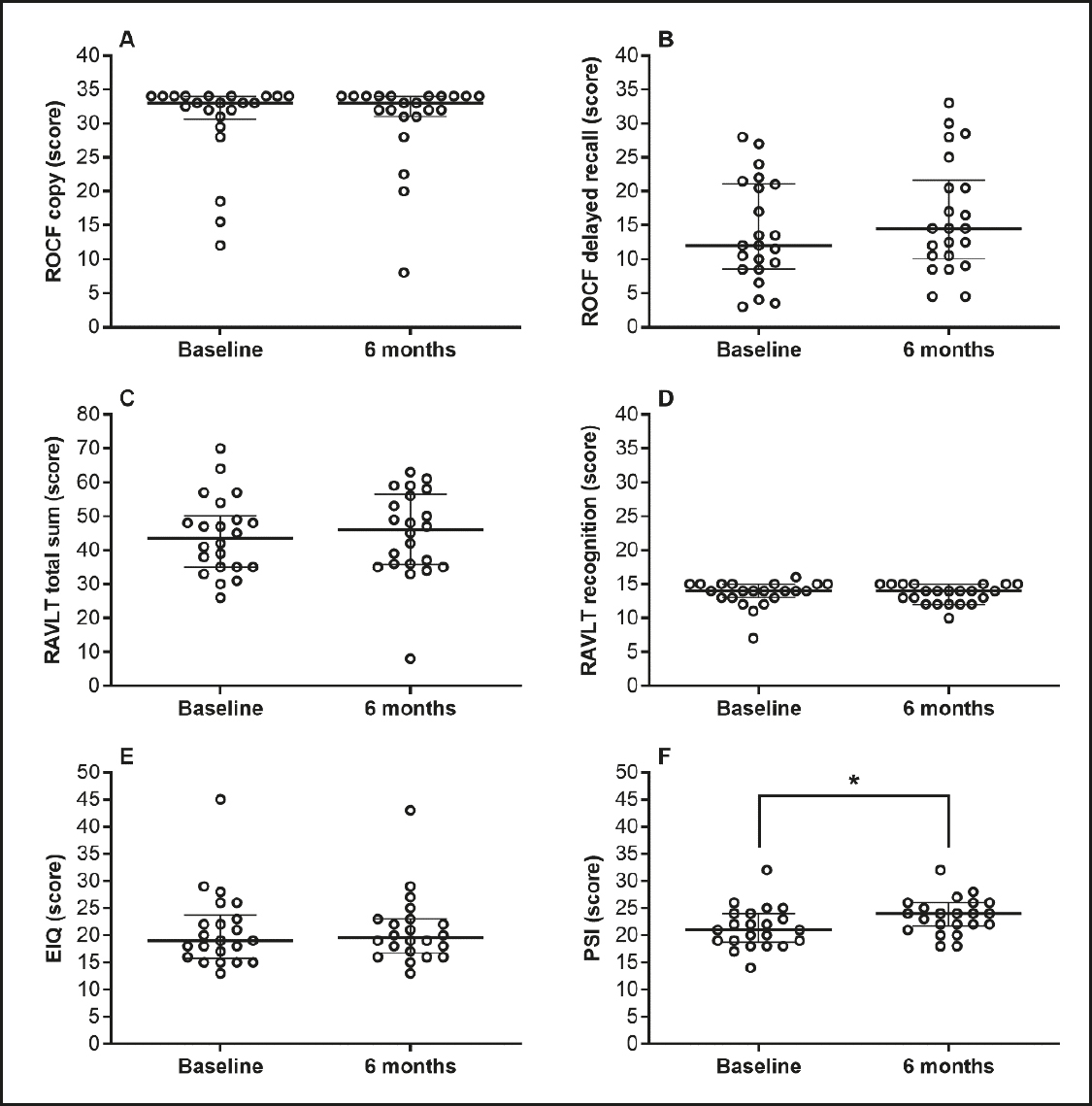
Figure 2. Visuospatial organization, planning, visual, short-term, long-term and episodic memory, verbal learning, retention of information, memory recognition. ROCF:: Rey-Osterrieth complex figure (panels A and B); RAVLT: Rey auditory-verbal learning test (panels C and D); EIQ: execution intelligence quotient (panel E); PSI: processing speed index (panel F). *Different from baseline for the Wilcoxon Rank-Sum test (p < 0.05). Data are median and interquartile range, n = 22.
The biomarkers decreased significantly (~ 41 % in GGT, 28 % in ferritin, 32 % in uric acid, 19 % in triglycerides, and 5.5 % in total protein). Moreover, we found a significant increase of folic acid (~ 108 %), high-density lipoprotein (HDL; ~ 9 %), and mean corpuscular volume (4 %) (Table III).
Table III. Biochemical and hematological data of obese patients before RYGB and 6 months after RYGB (n = 22)
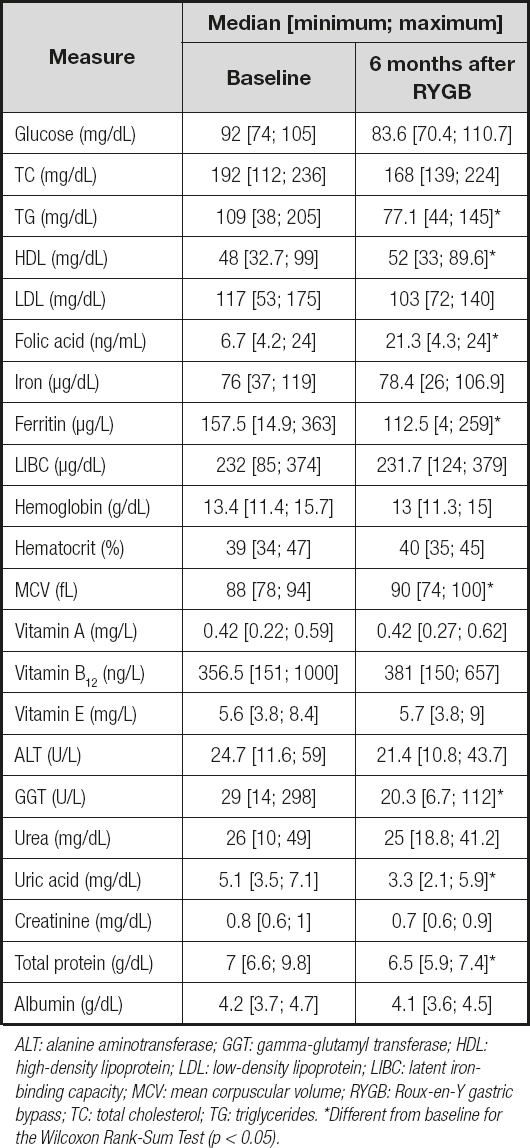
ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LIBC: latent ironbinding capacity; MCV: mean corpuscular volume; RYGB: Roux-en-Y gastric bypass; TC: total cholesterol; TG: triglycerides. *Different from baseline for the Wilcoxon Rank-Sum Test (p < 0.05).
A moderate positive correlation was observed between SCWT 2 and total protein (r = 0.75), and between PSI and body weight (r = 0.46) and GGT (r = 0.54, respectively) (Table IV).
Table IV. Correlations of biomarker changes with neuropsychological test changes achieving significant statistical differences at 6 months after RYGB
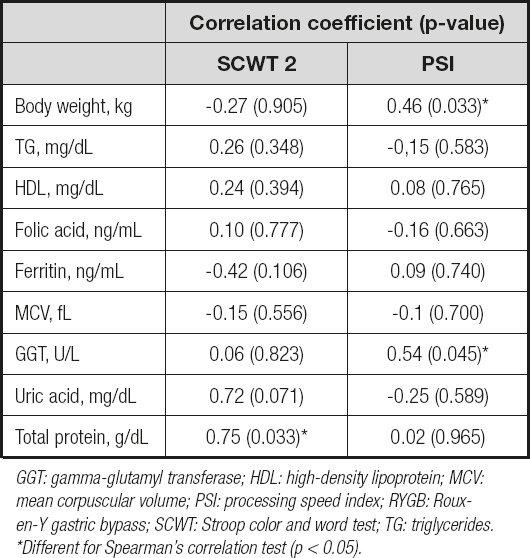
GGT: gamma-glutamyl transferase; HDL: high-density lipoprotein; MCV: mean corpuscular volume; PSI: processing speed index; RYGB: Rouxen- Y gastric bypass; SCWT: Stroop color and word test; TG: triglycerides. *Different for Spearman's correlation test (p < 0.05).
DISCUSSION
According to the applied neuropsychological tests, the participants of this study did not present cognitive impairments but performed poorly within the standard range in the preoperative assessments. The weight loss achieved in the 6-month postoperative period was accompanied by a slight increase in attention, mental flexibility, inhibitory control, and processing speed. Additionally, we observed a significant positive correlation between SCWT 2 and total protein, and between PSI and body weight, and PSI and GGT.
Unlike previous studies, our results did not indicate a cognitive deficit in bariatric surgery candidates (20). Regardless, the significant improvement in SCWT 2 in our study is in accordance with the improvements in attention switching 12 weeks after surgery (12). A previous study showed that the enhanced attention and mental flexibility persist for up to 24 months after bariatric surgery (9,21). The cognition prejudice in obesity is attributed to lower metabolic activity and a smaller gray matter volume in the prefrontal cortex, hypothalamus, and cingulate gyrus (22). Although the data are inconsistent, inflammatory cytokines released from visceral fat such as interleukin 6, tumor necrosis factor-alpha, and CRP lead to changes in both gray and white matter volumes (22).
It has also been shown that long-term obesity may lead to hippocampal damage, microvascular remodeling, and reduced frontal lobe volume, and interferes in brain maturation during adolescence (23). Our results did not show changes in short- or long-term, episodic, work, or visuospatial memory. Although our sample included nine patients who developed obesity in childhood or adolescence, it is possible that we did not observe cognitive deficiencies due to our insufficient sample size.
Improvement in processing speed was moderately associated with body weight and GGT. Although the mechanisms involved are not fully understood, GGT is a strong independent predictor of obesity (odds ratio of 4.8) (24). Similarly, the specific mechanism involving processing speed and GGT has not been described; however, GGT has recently been associated with cognitive decline risk in middle-aged and elderly persons (25). GGT was also proposed to trigger pro-oxidant and pro-inflammatory properties, which might affect the vascular structure (26).
Our GGT and ALT results were similar to those of a previous study regarding patients with obesity submitted to RYGB (27). Additionally, lower GGT levels may indicate reduced NAFLD inflammation (3). Although the mechanisms involved in NAFLD improvement after bariatric surgery are not fully understood, reduction in insulin resistance and dyslipidemia may contribute to it (27).
Uric acid (UA) reduction is consistent with other study that reported UA decrease in patients with obesity who underwent RYGB (28). The decrease in UA is proportional to the degree of weight loss (assessed by change in BMI). This relationship may be due to reduced urate production and increased renal urate clearance following weight loss (29). Further research is needed to clarify the origin and relationship between UA and adiposity. We also found a significant increase in HDL at the 6-month follow-up visit, and it is suggested that after RYGB, the subsequent hepatic expression of the paraoxonase-1 gene contributes to antioxidative and antiatherogenic HDL activity (30).
Our study's primary limitation was its small sample size, which restricted our understanding of the impact of postoperative weight loss on cognitive function. A secondary limitation was the lack of a control group, given we recruited the patients by convenience without randomization, which diminished the study's power. Thus, we were unable to determine causality for the observed cognitive changes induced by weight loss.
Additionally, a third limitation was the significant variability in our sample. The hospital environment, the researcher-participant relationships, and the patients' emotional aspects may have negatively influenced the collection of cognitive function data. In our study, the leading investigator was a psychologist who observed qualitative changes in the patients' affective, marital, familiar, and self-image domains. A fourth limitation we did not anticipate was modifiable risk factors such as low serum iron, folic acid, and vitamins. Thus, we are unsure whether nutrient supplementation prior to bariatric surgery could affect neurocognitive function. Lastly, the 6-month follow-up limited the substantiation of greater cognitive function improvements, such as those found in studies with 24 or 36 months after surgery (9,21).
To our knowledge, our study was the first one to analyze the association between cognitive function and nutritional biomarkers. However, we were unable to validate if an improvement in nutritional profile directly affects cognitive function. Further clinical studies, with a larger sample size, are necessary to understand the influences of nutritional status and massive weight loss over the cognitive function of bariatric patients. Additionally, bariatric patient difficulties must be considered when adapting the multidisciplinary approach, positively changing sedentary behavior and eating patterns.
As a practical application of the present results, this study could help guide the psychologist during follow-up of a bariatric patient. It is worth paying attention to the results of the cognitive analysis, as changes in it could signal nutritional deficiencies and, therefore, the patient should be referred for nutritional care, which reinforces the importance of a multidisciplinary team in the care of these patients.
In conclusion, after bariatric surgery the patients presented a slight improvement in attention, mental flexibility, speed processing, and several nutritional biomarkers. Nevertheless, bariatric surgery marginally impacted other cognitive domains, such as short- and long-term memory and language.












 Curriculum ScienTI
Curriculum ScienTI

